D1.3 Chemical Symbols, Formulas, and Equations
It is convenient to deal with macroscopic properties and atomic-scale models by defining symbols to represent elements, compounds, atoms, molecules, and structures. For example, the symbol Li can represent the element lithium or it can represent a lithium atom. When Li is used to represent the element, it should bring to mind various properties: Li is a metal that can be cut with a knife; Li reacts fairly quickly with air; Li reacts vigorously with water. When Li is used to represent a lithium atom, we can use numbers to indicate how many Li atoms are present. For example, 2 Li represents two lithium atoms. In Li2O, the formula for one substance that forms when lithium reacts with oxygen, the subscript “2” indicates that there are two Li atoms for every one O atom. Li2O also represents the macroscopic substance lithium oxide, which has specific properties including high melting point (1438 °C) and high solubility in water.
Symbols can also represent chemical reactions. When lithium reacts with water the chemical equation is
The letters in parentheses (s, ℓ, g, and aq) indicate that lithium, water, hydrogen, and lithium hydroxide are solid, liquid, gas, and an aqueous solution (a solution in which the solvent is water). The chemical symbols and formulas indicate that lithium and water are reactants and hydrogen and lithium hydroxide solution are products. The coefficients indicate how much of each reactant reacts away and how much of each product forms. The quantities can be expressed on the atomic scale as two lithium atoms reacting with two water molecules to give one hydrogen molecule and two lithium ions and two hydroxide ions in solution. (Lithium hydroxide consists of lithium ions, each with one unit of positive charge, and hydroxide ions, each with one unit of negative charge.) The quantities can be scaled up by a factor of 6.02214076 × 1023 (Avogadro’s number) and the equation says that two moles of solid lithium reacts with two moles of liquid water to give one mole of gaseous hydrogen and two moles of lithium hydroxide dissolved in water.
For molecules, symbolism can indicate which atoms are bonded to which other atoms, what types of bonds are present, and how the atoms are arranged in three-dimensional space. These structures and an animation illustrate some of the possibilities:
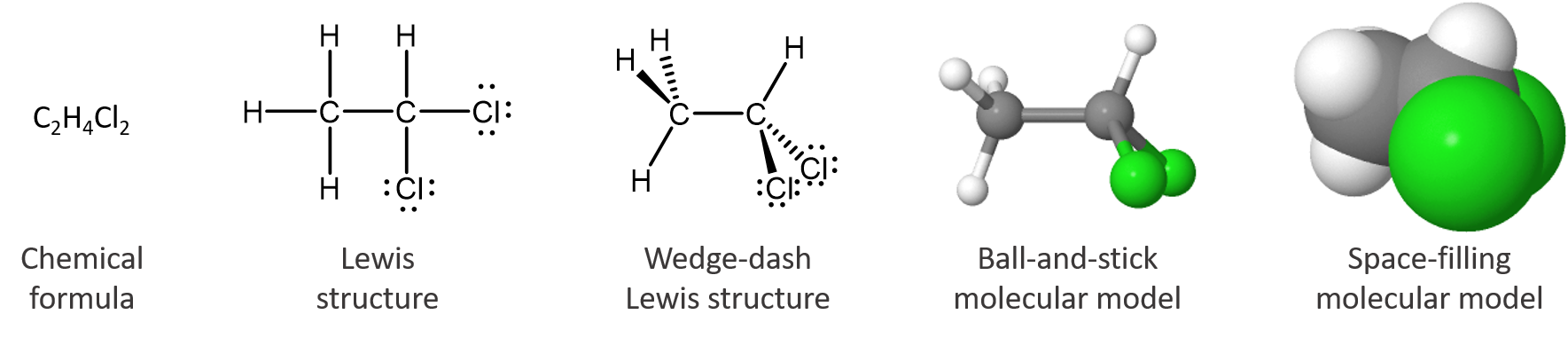
The chemical formula tells only the number of each kind of atom in the molecule. The Lewis structure indicates which atoms are bonded to which. The wedge-dash structure indicates that the molecule is three-dimensional, which is shown more clearly in the ball-and-stick model. In the space-filling model the sizes of all atoms and the molecule as a whole are shown; it is clear that a chlorine atom is bigger than a carbon atom, which is bigger than a hydrogen atom. The interactive, 3D model allows inspection of the molecule from any angle (use the mouse to change your viewpoint). The last three models are more pictorial than symbolic, but they are still representations of something we cannot see. None of these representations is the molecule itself; all provide useful information about its properties.
Chemists use representations such as these all the time and move effortlessly from one to the other as they think about molecules. Some imagination and much experience using these symbolic representations will enable you to make predictions about properties of substances and chemical reactions.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

