Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 10 Pre-Class Podia Activity
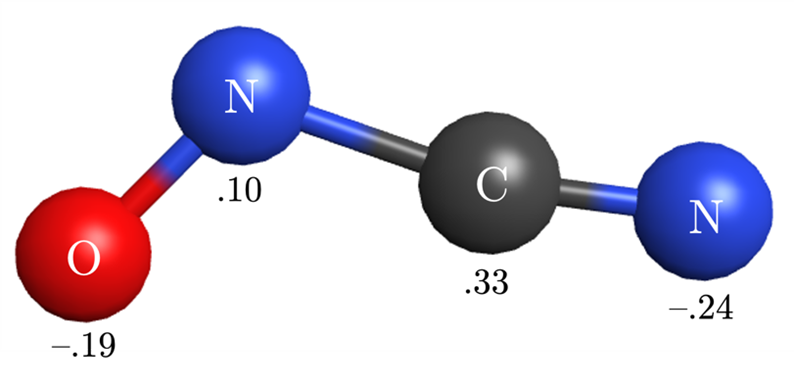
Below is a ball-and-stick representation of the molecule CN2O; the partial charges on each atom are indicated. Using your knowledge of electronegativity, construct a model of the electron distribution in this molecule that explains the relative values of these partial charges. Your model and explanation should address:
- why some atoms have partial positive charges, and why some have partial negative charges;
- why the nitrogen atoms have not only different charges, but charges of opposite sign;
- why the oxygen atom does not have the most negative charge, even though it is the atom of highest electronegativity in this molecule; and
- why these charges are not those predicted if you computed the formal charge on each atom.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

