D7.5 Formal Charge
It is useful to consider how valence electrons are distributed in a molecule. Formal charge, the charge an atom would have if the electron density in the bonds were shared equally between the atoms, is one way to do this. For an atom in a Lewis structure, half of the electrons in bonds are assigned to that atom, and all lone-pair electrons (which are not shared with other atoms) are assigned to that atom.
An atom’s formal charge is calculated as the difference between its number of valence electrons (in the unbonded, free neutral atom) and its assigned number of electrons in the molecule:
- If the assigned number of electrons equals the number of valence electrons, the atom has zero formal charge.
- If the assigned number of electrons exceeds the number of valence electrons, the atom has a negative formal charge.
- If the assigned number of electrons is less than the number of valence electrons, the atom has a positive formal charge.
Because formal charge accounts for all valence electrons in a molecule, the sum of the formal charges of all the atoms in a molecule/ion must equal to the actual charge of the molecule/ion. However, The formal charge for any given atom in a molecule is not the same as the atom’s actual partial charge in the molecule. This is because formal-charge calculations assume all covalent bonds are nonpolar, which is seldom the case except for homonuclear molecules. We will discuss bond polarity in further detail in unit 2.
Exercise: Formal Charge from Lewis Structure
Exercise: Formal Charge from Lewis Structure
Using Formal Charge to Predict the Most Likely Lewis Structure
While formal charges do not portray the true electron density distribution within a molecule, they nonetheless account for electron arrangement in a Lewis structure in units of whole electron. Therefore, if following the steps for drawing Lewis structures leads to more than one possible arrangement of electrons and/or atoms for a given molecule, formal charges can help to decide which arrangement is likely to be the most stable, and hence the most likely Lewis structure for the given molecule.
- For an uncharged molecule, a Lewis structure in which all atoms have a formal charge of zero is preferable.
- The fewer atoms with nonzero formal charges, the better.
- The smaller the magnitude of the formal charges, the better.
- A Lewis structure with formal charges of the same sign (both + or both −) on adjacent atoms is less likely.
- Lewis structures with negative formal charges on more electronegative atoms are preferable. (We will discuss electronegativity in more detail in Unit 2; the electronegativity trend for second row elements are: Li < Be < B < C < N < O < F, with F being most electronegative.)
For example, consider these three possible Lewis structures of carbon dioxide, CO2:
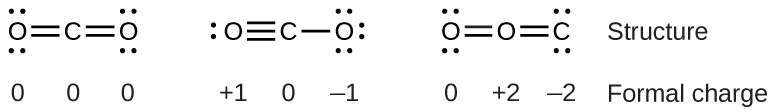
All structures have octets on each atom, but the structure on the left is the best Lewis structure for CO2 because all atoms have zero formal charge. The structure on the right is least likely because of the larger formal charges.
Exercise: Formal Charge and Lewis Structure
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

