D10.6 Formation of σ and π Bonds
Formation of a σ bond
When a σ bond forms between two atoms (A and B), a hybrid orbital with one unpaired electron from atom A overlaps with a hybrid orbital with one unpaired electron from atom B. The resulting σ bond is an orbital that contains a pair of electrons. This is similar to drawing a Lewis structure, where a bond pair (single covalent bond) is formed using one electron dot from each atom. For example, Figure: Hybrid-orbital Overlap shows the formation of a C-C σ bond from two sp3 hybridized carbon atoms.
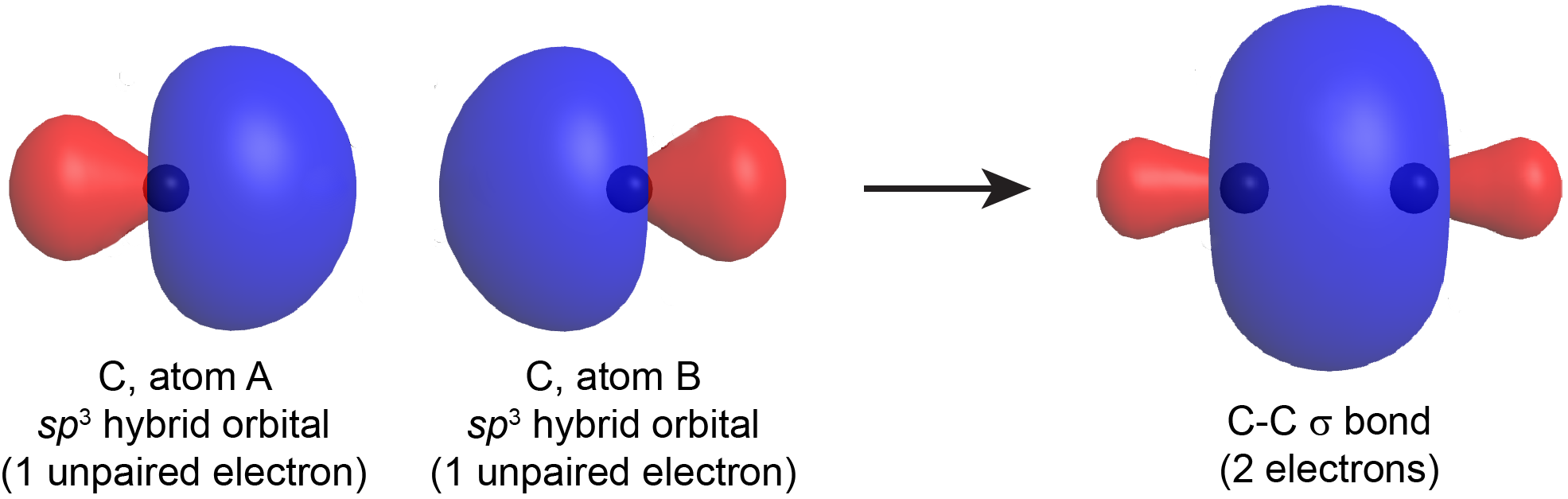
A σ bond can be formed by overlap of any type of hybrid orbital on atom A and any type of hybrid orbital on atom B; many σ bonds involve an sp2 or sp hybrid on one atom or the other. The σ bond formed by two hybrid orbitals (valence bond theory) is similar to a σ bond formed in a diatomic molecule as described by molecular orbital theory. However, in valence bond theory, the σ bonds are always described individually between each pair of bonded atoms, even in polyatomic molecules with more than three atoms. This is not the case for molecular orbital theory.
Notice that in both valence bond theory and molecular orbital theory, the σ bond has a cylindrical symmetry with respect to the bonding axis. If you rotate the bond around the internuclear axis, the shape of the electron density of the σ bond does not change.
Formation of a π bond
In ethene, C2H4, which contains a C=C double bond, each carbon atom is sp2 hybridized. Around each C atom there are three bonds in a plane: two of the sp2 hybrid orbitals form two C–H σ bonds and the third sp2 hybrid orbital forms a C-C σ bond.
The C=C double bond also includes a π bond, which is formed from the overlap of the unhybridized 2p atomic orbital on each carbon atom. The unhybridized 2p atomic orbital is perpendicular to the plane of the sp2 hybrid orbitals (see Figure: π Bond Formation). Thus when the 2p atomic orbitals overlap in a side-by-side fashion to form a π bond, the electron densities in the π bond are above and below the plane of the molecule (the plane containing the σ bonds).
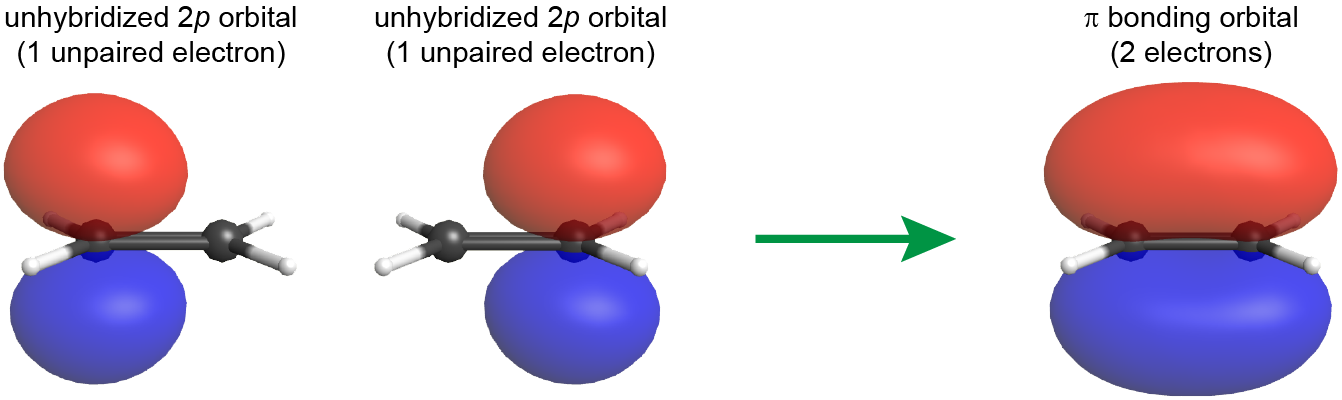
Because the π bond is formed from unhybridized atomic orbitals, it is quite similar to π bonds described by molecular orbital theory, even for polyatomic molecules.
What if the plane containing the sp2 hybrid orbitals of one carbon atom were to rotate 90° relative to the other carbon atom? In that case, the two 2p atomic orbitals would also be oriented at 90° to each other (see Figure below); the 2p atomic orbitals no longer overlap side-by-side and the π bond cannot form.
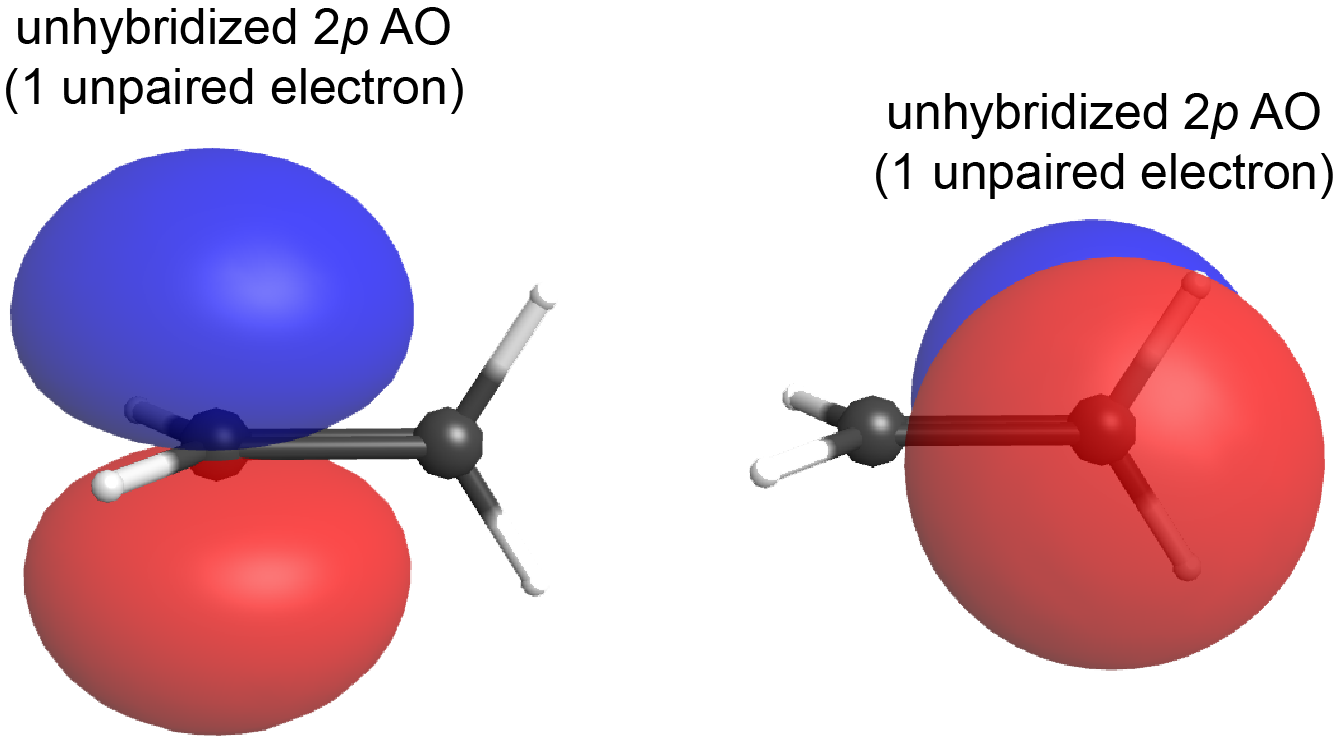
This is a significant difference between σ and π bonds: one atom rotating around the internuclear axis with respect to the other atom does not change the extent to which the σ bonding orbitals overlap because the σ bond is cylindrically symmetric about the bond axis (see Figure: Hybrid-orbital Overlap); in contrast, rotation by 90° about the internuclear axis breaks the π bond entirely because the p atomic orbitals can no longer overlap.
In acetylene, H−C≡C−H, each carbon atom is sp hybridized with two unhybridized 2p atomic orbitals. One sp hybrid orbital from each C atom overlaps to form a C-C σ bond, the other sp hybrid orbital forms a C-H σ bond with a hydrogen atom. The unhybridized 2p atomic orbitals overlap to form two perpendicular C-C π bonds (Figure: Bonds in HCCH). The two carbon atoms of acetylene are thus bound together by one σ bond and two π bonds, giving a triple bond.
Brief Summary
- Hybrid orbitals are derived by combining two or more atomic orbitals from the valence shell of a single atom.
- Atomic orbitals are the most stable arrangement of electrons in isolated atoms.
- Hybrid orbitals are important in molecules because they result in stronger (lower energy) σ bonding.
- Most σ bonds are formed from overlaps of hybrid orbitals. Most π bonds are formed from overlap of unhybridized atomic p orbitals.
- The number of hybrid orbitals equals the number of valence atomic orbitals that were combined to produce the hybrid orbitals.
- Because π bonds are formed from unhybridized p atomic orbitals, an atom that is involved in π bonding cannot be sp3 hybridized.
- If a hybridized orbital on an atom in a molecule has a pair of electrons but is not pointing at another atom, the filled hybrid orbital is not involved in bonding. This corresponds to a lone pair on an atom in a Lewis structure.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

