D28.6 Gibbs Free Energy and Coupled Reactions
When we talk about the thermodynamics of a reaction, we are concerned primarily with the difference in energy between reactants and products. When the Gibbs free energy of the products is lower than that of the reactants, a reaction is said to be exergonic. Conversely, an endergonic reaction is one in which the products are higher in Gibbs free energy than the reactants.
The decrease in Gibbs free energy of an exergonic reaction corresponds to the maximum useful work that can be done by the reaction system. Conversely, the increase in Gibbs free energy of an endergonic reaction tells you what is the minimum work that must be done on the system to force the reaction to occur.
Exercise: Gibbs Free Energy and Work
In the multi-step ozone decomposition reaction discussed in the previous chapter, step 1 of the reaction mechanism is endergonic while step 2 is exergonic. Overall, the 2 O3(g) ⟶ 3 O2(g) reaction is a product-favored reaction under standard state conditions, despite step 1 being reactant-favored. This is because step 2 is much more product-favored than step 1 is reactant-favored, and the overall summed ΔrG° is negative.
We can apply this idea to otherwise separate reactions: we can drive a reactant-favored process to occur if we couple it with a reaction that is product-favored. For example, consider the recovery of aluminum from alumina (Al2O3), which is refined from bauxite ore:
This is a multi-step reaction, and at least 1576.4 kJ is needed to change 1 mole of Al2O3(s) into 2 moles of Al(s) and 1.5 moles of O2(g) (at 1 bar). In a modern aluminum manufacturing plant, this energy is supplied electrically and the electricity is often provided by burning coal. Assuming coal to be mainly carbon, the combustion reaction is:
Thus the ΔrG° values indicate that, under standard-state conditions and ideal 100% efficiency, at least four moles of carbon/coal must burn to process each mole of Al2O3 ore. (In practice the aluminum smelting process is only 17% efficient, so it is necessary to burn nearly 6 times the theoretical amount of coal.) Coupled reactions occur simultaneously and there is a means of exchanging energy between them. The energy exchange occurs via the electric power grid in this specific case.
In other words, a reaction that is endergonic under standard-state conditions can be coupled to a separate exergonic reaction that drives the endergonic reaction (the thermodynamically unfavorable one) to occur. The ΔrG° values for the two coupled reactions are summed to yield the overall ΔrG°. For the alumina example, multiply the carbon combustion reaction by 4, add the two reaction equations, and apply Hess’s Law; this gives:
The overall reaction now has a negative ΔrG° and is product-favored.
Under nonstandard-state conditions, a reaction with ΔrG < 0 can drive a reaction with ΔrG > 0, provided energy can be transferred from one to the other.
In a possibly familiar biological example, living organisms often couple the product-favored hydrolysis of ATP (adenosine triphosphate) to a reactant-favored reaction. Thus ATP hydrolysis reaction can be used to drive a necessary, but thermodynamically unfavorable, reaction.
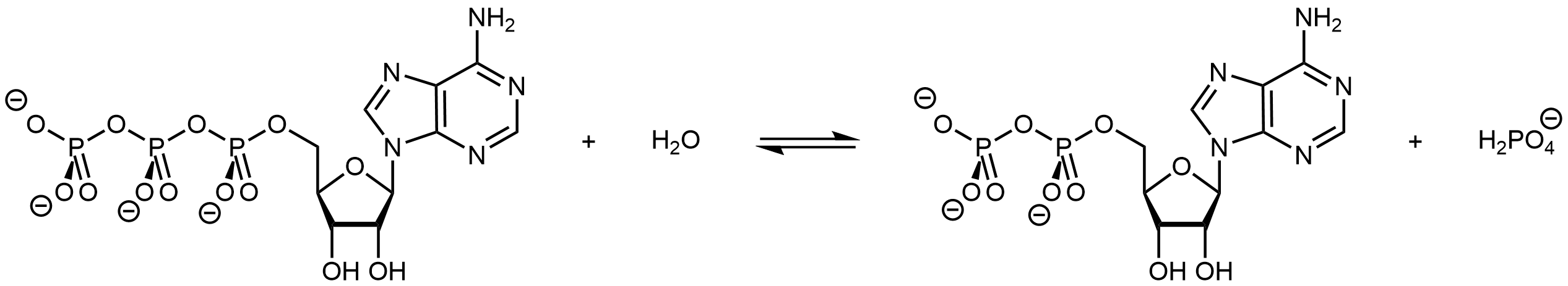
ATP can be made available in an organism where an endergonic reaction needs to occur. Its hydrolysis can then be coupled with the endergonic reaction to yield a thermodynamically favorable overall reaction.
Exercise: Characteristics of Exergonic Processes
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

