D6.2 Molecular Orbital (MO) Diagram
When two H atoms come together to form H2, both in-phase overlap and out-of-phase overlap of 1s atomic orbitals are possible and both occur. Hence, two 1s atomic orbitals (AOs) overlap to form two molecular orbitals (MOs). This is true in general: the number of MOs formed equals the total number of AOs from which they are derived.
A MO diagram shows the relative energies of MOs and AOs and indicates which MOs form from overlap of which AOs. For example, Figure: MO Energy Levels (below) shows the MO diagram of the H2 molecule. A MO lower in energy than the AOs from which it is derived is called a bonding MO. An electron occupying this bonding MO increases the stability of the bond (strengthens the bond). The H2 bonding MO is labeled “σ1s“; the subscript “1s” designates that it is derived from the 1s AOs.
A MO higher in energy than the AOs from which it is derived is called an antibonding MO—an electron occupying this MO decreases the stability of the bond (weakens the bond). The H2 antibonding MO is labeled as “σ*1s“; the “*” denotes that it is an antibonding MO.
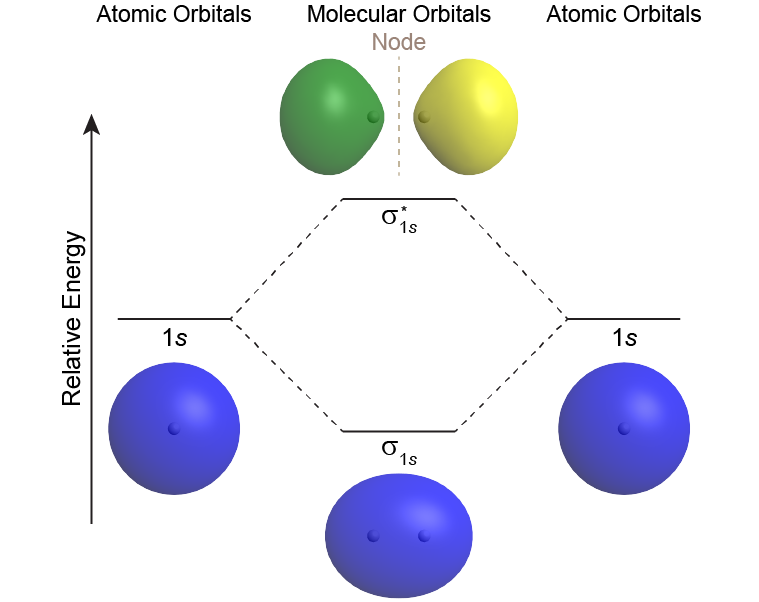
The Greek letter in the MO labels denotes the symmetry of the MO with respect to the internuclear axis (the line connecting the two nuclei). Greek letters σ, π, δ are analogous to the Roman letters s, p, d that designate shapes of atomic orbitals. A MO that is cylindrically symmetric is called a sigma MO and designated σ. (Cylindrically symmetric means that if you stood the molecule on end and spun it around the internuclear axis it would look the same all the time—just as a cylinder would look if you stood it on end and spun it).
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

