D14.6 Oxidation Reactions of Alcohols
All substances whose molecules contain hydrocarbon sections are combustible—the hydrocarbon parts can be oxidized completely to carbon dioxide and water. However, for many compounds, controlled oxidation is more important than combustion because it can convert one type of functional group into another functional group, giving us chemical compounds useful in various applications. An example is oxidation of alcohols, which can be used to convert alcohols to aldehydes, ketones, or carboxylic acids.
Oxidation of an organic compound can usually be recognized as either an addition of oxygen atom(s) to, or removal of hydrogen atoms from, the reactant molecule. The ease with which an alcohol can be oxidized and the extent of the oxidation depends on whether the alcohol is primary, secondary, or tertiary.
For a primary alcohol, controlled oxidation first produces an aldehyde, then the aldehyde is further oxidized to a carboxylic acid:
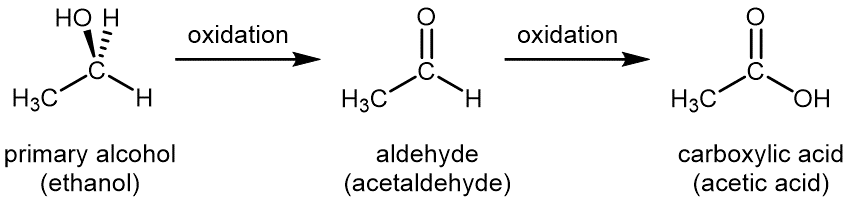
Common oxidizing agents used in the laboratory for controlled oxidation are aqueous solution of potassium permanganate, KMnO4(aq), or aqueous acidic potassium dichromate, K2Cr2O7(aq). A special oxidizing agent is needed to stop the reaction at the aldehyde step to yield aldehyde, rather than carboxylic acid, as the final product.
For a secondary alcohol, the oxidation product is a ketone:
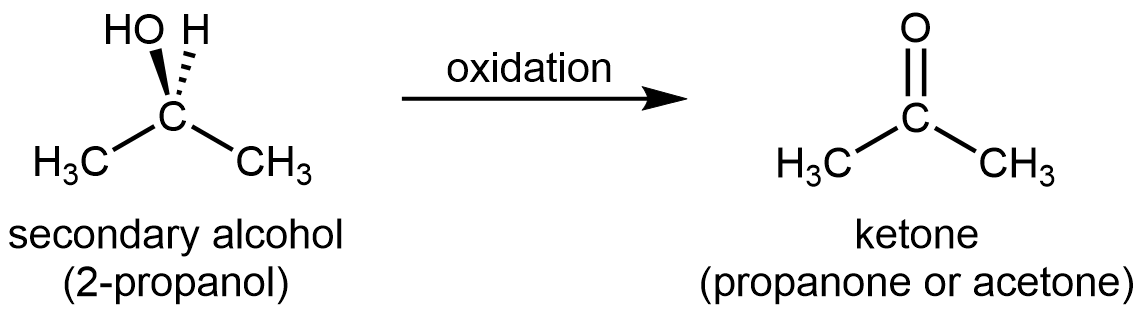
Ketones are difficult to oxidize further because there is no obvious way to add one more oxygen atom to the carbonyl carbon without breaking C–C bonds, and there are no hydrogen atoms to remove from that carbon either.
Tertiary alcohols, with no hydrogen atoms attached to the carbon atom that is bonded to the –OH group, are difficult to oxidize. Tertiary alcohols do undergo combustion (to yield CO2 and H2O), but they usually do not undergo controlled oxidation.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

