D23.3 Steric Factor
The steric factor is the fraction of collisions energetic enough to react that actually results in reaction. In the cis–trans isomerization example we were just discussing, not all cis molecules with energy greater than Ea will form the trans isomer. Only specific collisions, where one molecule hits a cis molecule in a way that causes one carbon to start rotating with respect to the other carbon, will push the cis molecule forward along the reaction progress.
For a more illustrative example of why some sufficiently energetic collisions do not result in a reaction proceeding forward, consider this reaction:
The reaction energy diagram is shown below. In order to proceed forward, an O2 molecule needs to collide with a CO molecule with sufficient energy to reach the transition state, where a new C=O double bond begins to form while the O=O double bond breaks and one of the C≡O π bonds also breaks.
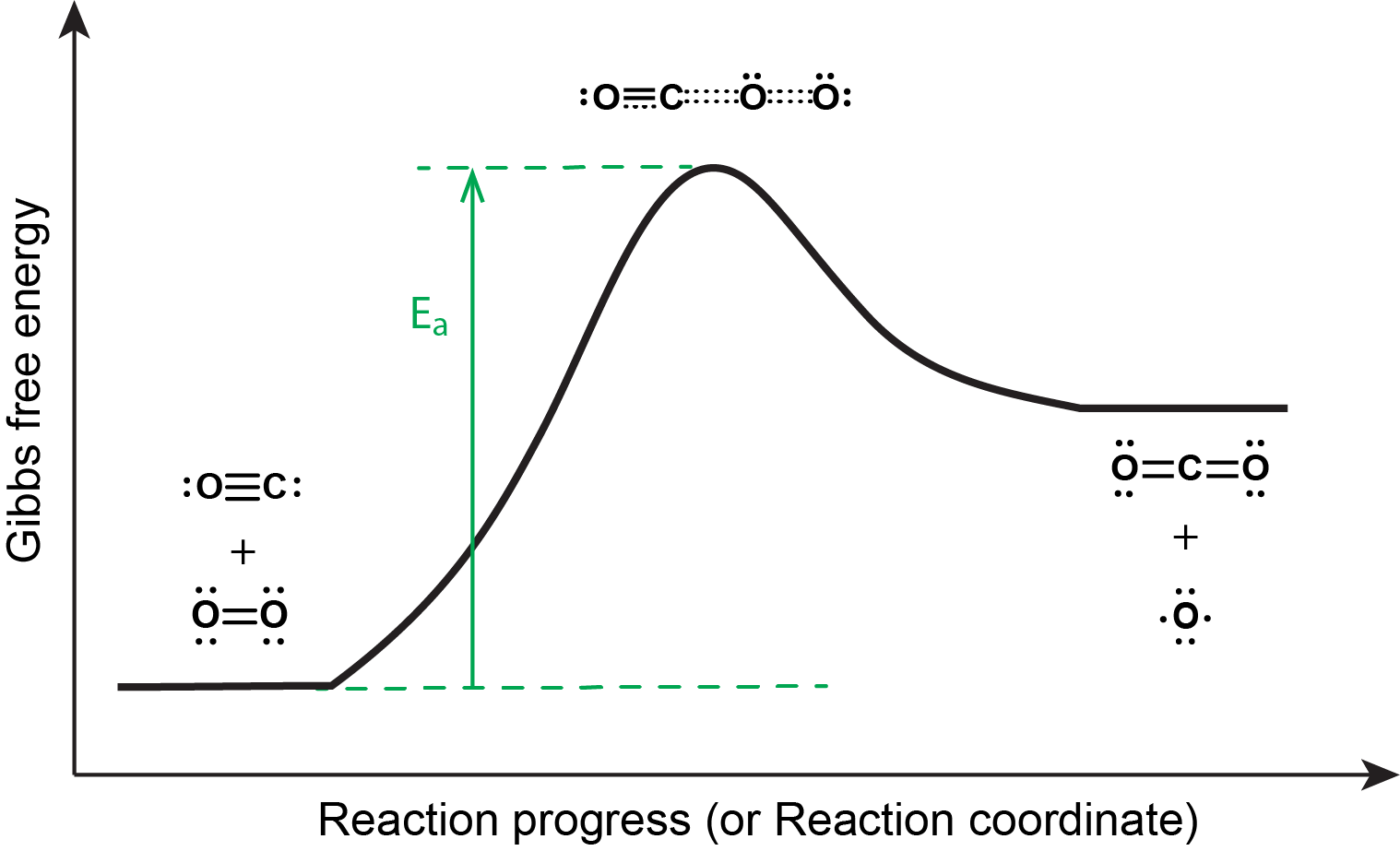
Although there are many different possible orientations a CO and an O2 molecule can have relative to one another, consider the two presented in the activity below.
Activity: Steric Factor
This relatively simple example illustrates how important the orientation of the collision is in terms of reaching the transition state and forming the desired product for a given reaction.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

