D40.4 Introduction to Voltaic Cells
A voltaic cell (or galvanic cell) is an electrochemical cell in which a spontaneous redox reaction produces an electric current. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate.
When the copper metal contacts the silver nitrate solution, silver metal begins to form on the copper surface and Cu2+ ions pass into the solution (indicated by the blue-green color of the solution). The net ionic equation for the reaction is:
which may be split into its two half-reactions that sum to the overall reaction:
| Oxidation: | Cu(s) | ⟶ | Cu2+(aq) + 2 e‾ |
| Reduction: | 2 Ag+(aq) + 2 e‾ | ⟶ | 2 Ag(s) |
The half-reactions make clear that two electrons are transferred from a copper atom, which forms a Cu2+ ion. Each of the two electrons is then transferred to one Ag+ ion, which forms a silver atom. Because a flow of electrons constitutes an electric current, this electron transfer can generate an electric current if we devise a way to carry out the two half-reactions in separate vessels and connect them with a metal (electrically conductive) wire. This is in essence how a voltaic cell is designed. Figure: Voltaic Cell shows one way to do this.
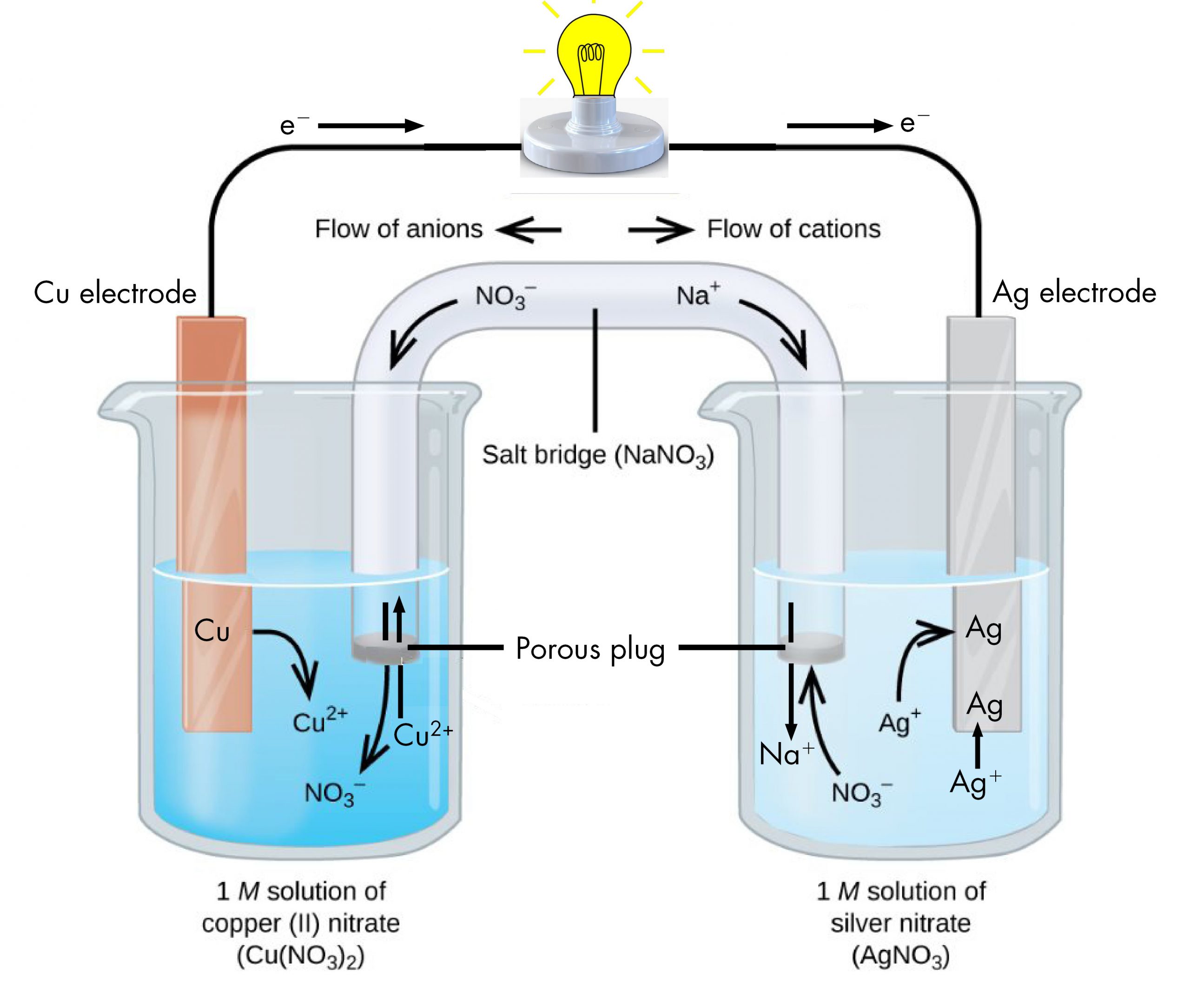
The beaker on the left contains a 1-M solution of copper(II) nitrate [Cu(NO3)2] with a strip of copper metal partially submerged in the solution. The copper strip is an electrode, a means for conducting electrons into or out of the solution. At the surface of the copper strip, the oxidation half-reaction occurs:
The flow of electrons (electric current) passes out of the solution via the copper strip, flows through the light bulb, and moves into the silver strip in the beaker on the right. In the right-hand beaker, the reduction half-reaction occurs near the surface of the silver strip:
Thus, with the two half-reactions occurring in separate beakers, an electric current can be generated. The container in which each half-reactions occurs is called a half-cell.
If this were all that happened, the electric current would not flow for long. In the left-hand beaker, one Cu2+ ion is added to the solution for every two electrons conducted into the external wire. This means that the solution is continuously accumulating excess positive ions as the reaction occurs and an electric charge is building up. Such a charge build up would prevent further oxidation reaction from occurring. This can be mitigated if some positive ions move out of the beaker or some negative ions move into the beaker. A similar issue is occurring in the right-hand beaker, where Ag+ ions are being removed from the solution. Balancing total ionic charges requires either negative ions move out of the right-hand solution or positive ions move in.
This balancing of ion charges in the two separated half-reactions is maintained by the salt bridge, a solution of a salt that does not mix with either half-cell solution but allows ions to pass into or out of the half-cells. By allowing ions to conduct charge into or out of the half-cells, the salt bridge completes the electrical circuit involving the two half-cells. Without it, current cannot flow for more than an instant. Notice that negative ions in the salt bridge move in the same direction as electrons around the circuit (clockwise in the above figure) and positive ions move in the opposite direction.
The half-cell in which oxidation occurs is called the anode. The half-cell in which reduction occurs is called the cathode. It is easy to remember that the anode involves oxidation because both words begin with a vowel. It is easy to remember that the cathode involves reduction because both words begin with a consonant. These definitions, anode/oxidation and cathode/reduction, are true for any electrochemical cell, not just a voltaic cell.
Exercise: Reactions for a Voltaic Cell
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

