D15.2 Addition Polymers
Addition polymers are made by addition reactions, where two molecules combine to form a single product molecule (we have already seen examples of addition reactions involving alkenes). Typical monomers for addition polymerization have at least one C=C double bond.
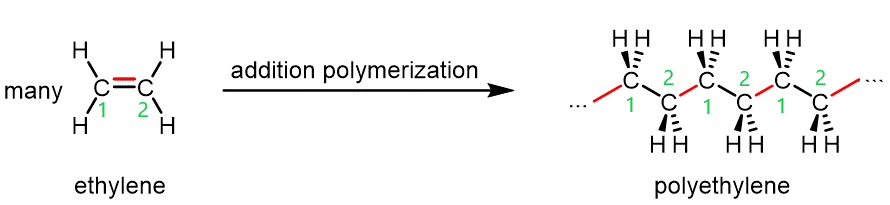
The figure below shows polyethylene, an addition polymer, forming from ethylene (ethene, H2C=CH2) monomers.
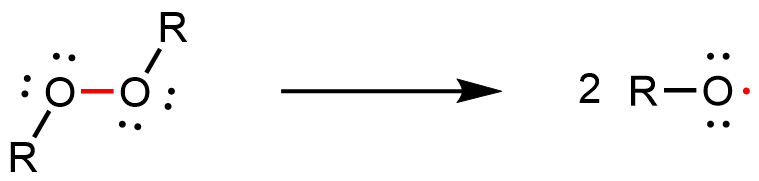
The addition polymerization reaction can be initiated by a molecule with an unpaired electron—a free radical. An example initiator is an organic peroxide, which can form two free radicals when the relatively weak O-O bond breaks:
Activity: Analyzing Peroxide Decomposition
Suppose you were asked to provide evidence that the O-O bond in an organic peroxide is relatively weak. You are told by another student that the O-O bond in, for example, diethyl peroxide, CH3CH2O-OCH2CH3, breaks at a lower temperature than other bonds in the molecule. Write a description of how you could verify (or refute) this student’s statement.
Write in your notebook, then left-click here for an explanation.
When a radical encounters an ethylene monomer, the ethylene π bond breaks. One electron from the π bond goes to pair with the electron from the radical and form a σ bond. The other electron from the π bond remains a radical, and can go on to react with another ethylene monomer.
The process illustrated in the figure above repeats with many, many more monomers, sometimes as many as 100,000 units, yielding a long polymer chain of carbon atoms.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

