D14.3 Aldehydes, Ketones, Ethers, Esters
Aldehydes
An aldehyde or a ketone contains a carbonyl group, a carbon atom double bonded to an oxygen atom. The carbon atom in a carbonyl group is called the carbonyl carbon. In an aldehyde functional group, the carbonyl carbon is also bonded to a hydrogen atom. Hence, an aldehyde group can at most bond to one R group, and the aldehyde group is always at the end of a chain of carbon atoms.
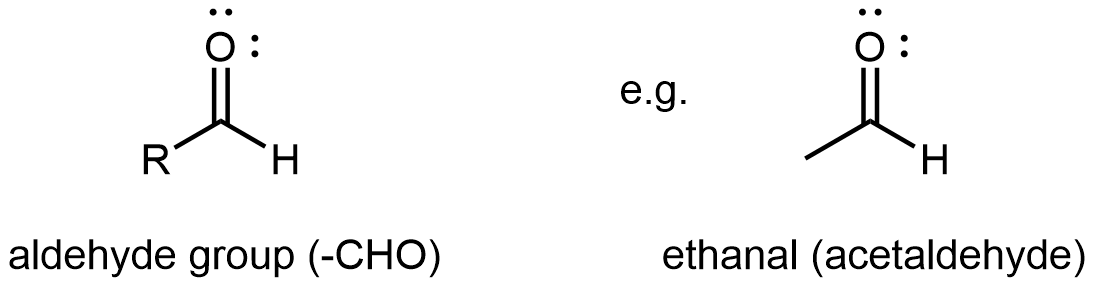
Ketones
A ketone functional group consists solely of the carbonyl group. It bonds to two R groups, which may be the same or different, and is found partway along a chain of carbon atoms.
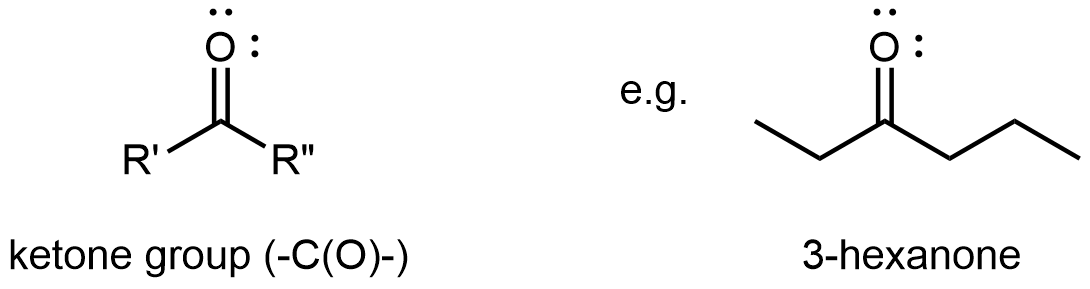
The reactivity of both aldehydes and ketones are directly related to the reactivity of the carbonyl group.
Formaldehyde, the simplest aldehyde, is a colorless gas with a pungent and irritating odor. It is sold in an aqueous solution called formalin, which contains about 37% formaldehyde by mass.
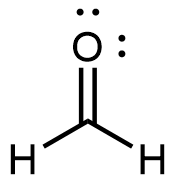
Formaldehyde causes coagulation of proteins, so it kills bacteria (and any other living organism) and stops many of the biological processes that cause tissues to decay. Thus, formaldehyde is used for preserving tissue specimens and embalming bodies. It can also be used to sterilize soil or other materials. Formaldehyde is used in the manufacture of Bakelite, a hard plastic having high chemical and electrical resistance.
Dimethyl ketone, CH3COCH3, commonly called acetone, is the simplest ketone. It is a colorless liquid that can be made commercially by fermenting corn or molasses. Among its many uses are: as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics, and varnishes; as a paint and varnish remover; and as a solvent in the manufacture of pharmaceuticals and chemicals.
Exercise: Ketone Hybridization and Local Bond Geometry
Ethers
An ether functional group contains the group –O–, which bonds to two different R groups and is found in the middle of a molecule.
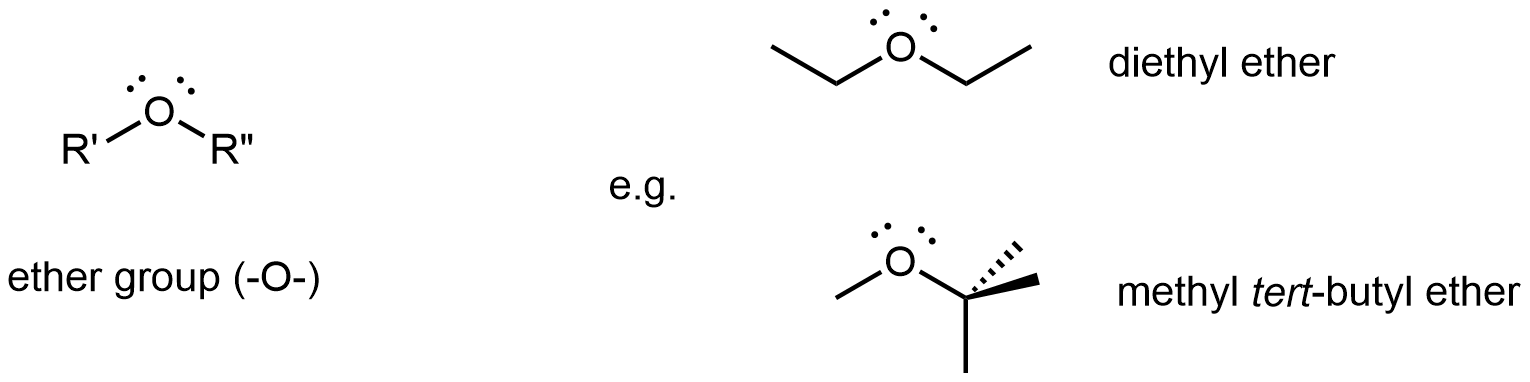
Diethyl ether, the most widely used ether, is a colorless, volatile liquid that is highly flammable. It was first used in 1846 as an anesthetic, but better anesthetics have now largely taken its place. Diethyl ether and other ethers are now used primarily as solvents for gums, fats, waxes, and resins. Methyl tert-butyl ether (abbreviated MTBE) is used as an additive for gasoline. MTBE belongs to a group of chemicals known as oxygenates due to their capacity to increase the oxygen content of gasoline.
Exercise: Ether Hybridization and Local Bond Geometry
Esters
An ester functional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. A general ester structure has an R group bonded to the carbonyl carbon atom and another R group bonded to the second oxygen. It is a functional group that is found in the middle of a molecule.
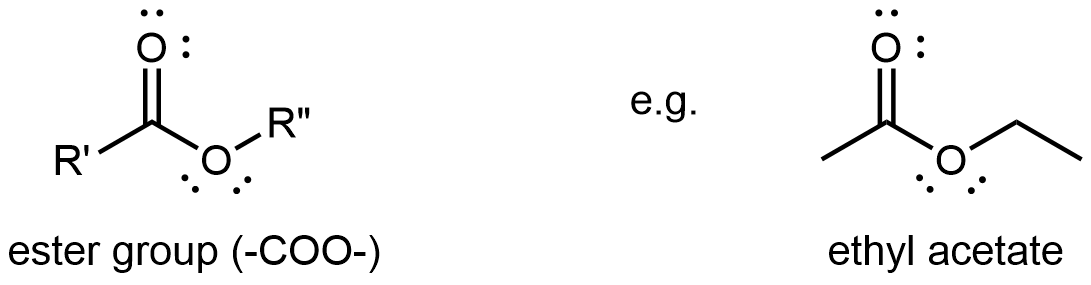
The ester functional group’s carbon atom is sp2 hybridized with a trigonal planar local geometry. Its carbonyl oxygen is sp hybridized, and one of its unhybridized 2p atomic orbitals forms the π bond with the carbon’s unhybridized 2p atomic orbital. This oxygen also has two lone pairs: one occupies a sp hybrid orbital; the other occupies a 2p atomic orbital that is perpendicular to the π bond. The second oxygen (non-carbonyl oxygen) is sp2 hybridized and has a bent local geometry. It also has two lone pairs, one in a sp2 hybrid orbital, the other in the unhybridized 2p atomic orbital.
As shown in the preceding figure, the 2p lone pair on the non-carbonyl O is aligned parallel to the 2p orbitals that form the π bond. This leads to some delocalization of the lone pair electron densities, which can be expressed by resonance structures:
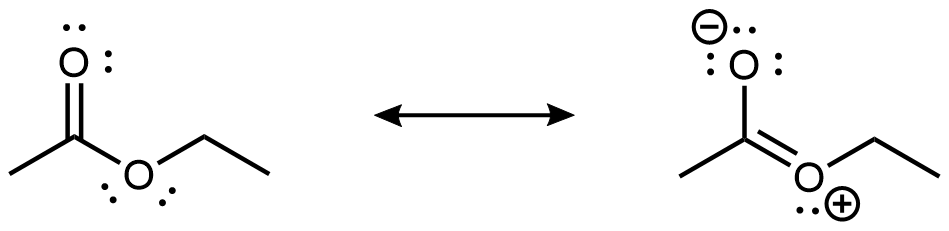
While the resonance structure on the right makes only a fairly minor contribution to the description of the ester molecule, that structure is helpful in understanding the ester’s chemical and physical properties. For example, the -COO- ester group is planar, and the non-carbonyl C-O bond is not as freely rotatable as a typical single bond. Moreover, an ester’s reactivity is quite different from that of a ketone or an ether, and hence an ester is a distinct functional group.
Activity: Ester Hybridization and Local Bond Geometry
The odors of ripe bananas and many other fruits are due to the presence of esters.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

