D10.1 Bonds, Molecules, and Structures
In a molecule, atoms are connected by covalent bonds that result from overlap of atomic orbitals. Molecules can consist of a small or large number of atoms, and may involve all the same kind of atom or a dozen different kinds of atoms. The atoms can be connected by bonds in a variety of different ways, so there is a broad range of different molecular structures. Thus, there are many things a chemist needs to know about a molecule:
- What kinds of atoms and how many of each are in the molecule?
- Which atoms are bonded to which other atoms?
- What are the bond lengths between atoms?
- How strong are the bonds?
- What are the angles between the bonds?
- How are the atoms arranged in three-dimensional space?
- What kinds of attractions are there between molecules?
- How strong are the attractions between molecules?
- Are there noncovalent attractive forces within a large molecule, holding one part of the molecule to another?
At this point we have introduced the first four of these, using molecular formulas, Lewis structures, atomic radii/bond lengths, and bond energies. For example, in a water molecule, the molecular formula H2O indicates that there are two H atoms and one O atom. A Lewis structure, H–O–H shows that both H atoms are bonded to the O atom. The O–H bond length (94 pm) and bond energy (467 kJ/mol) verify that the bonds are strong—separating the atoms is difficult.
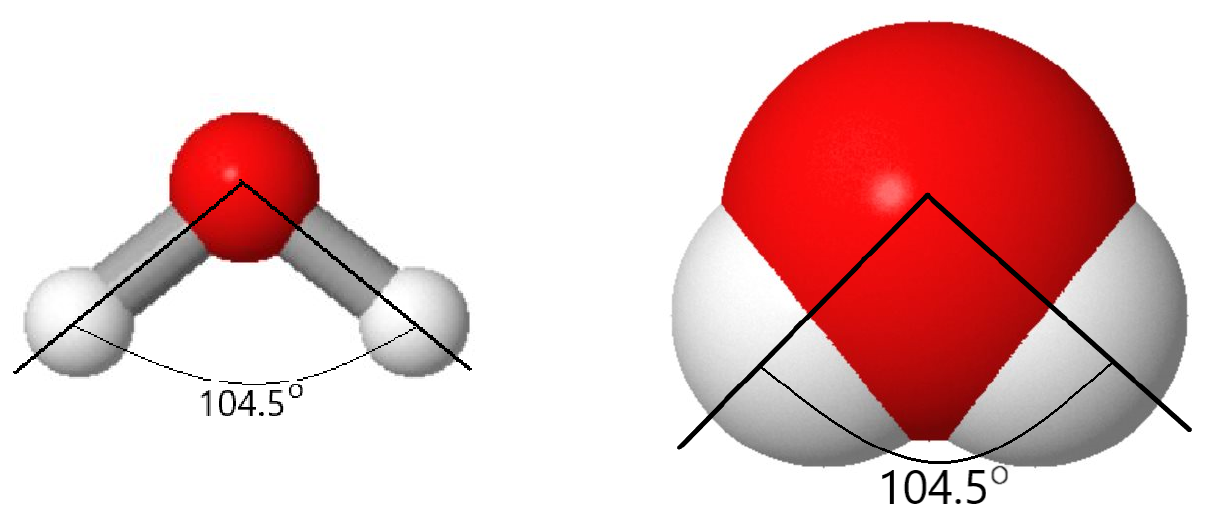
But there is more to a water molecule than that. Experimental and calculation results show that the angle between the two O–H bonds is 104.5°—somewhat more than a right angle. Angles larger or smaller than 104.5° result in higher energy (lower stability) for the molecule. As a result of the type of atoms in the water molecule and its molecular shape, there are stronger forces between water molecules than between methane (CH4) molecules, although both contain the same number of electrons.
As the number of atoms in a molecule increases, the last four factors in the list above become more and more important. You will learn about those factors throughout this Unit, beginning with additional properties of chemical bonds in the next sections.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

