D40.6 Cell Notation
Drawing a pictorial diagram, like the figure below, to define a voltaic cell takes a lot of time. Cell notation is an abbreviation that summarizes the important information about a voltaic cell. In cell notation, a vertical line, |, denotes a phase boundary and a double line, ||, a salt bridge. The anode electrode is written to the left, followed by the anode solution, then the salt bridge, then the cathode solution, and, finally, the cathode electrode to the right. Figure: Cell Notation below shows how the cell notation for a voltaic cell relates to various components of the cell.
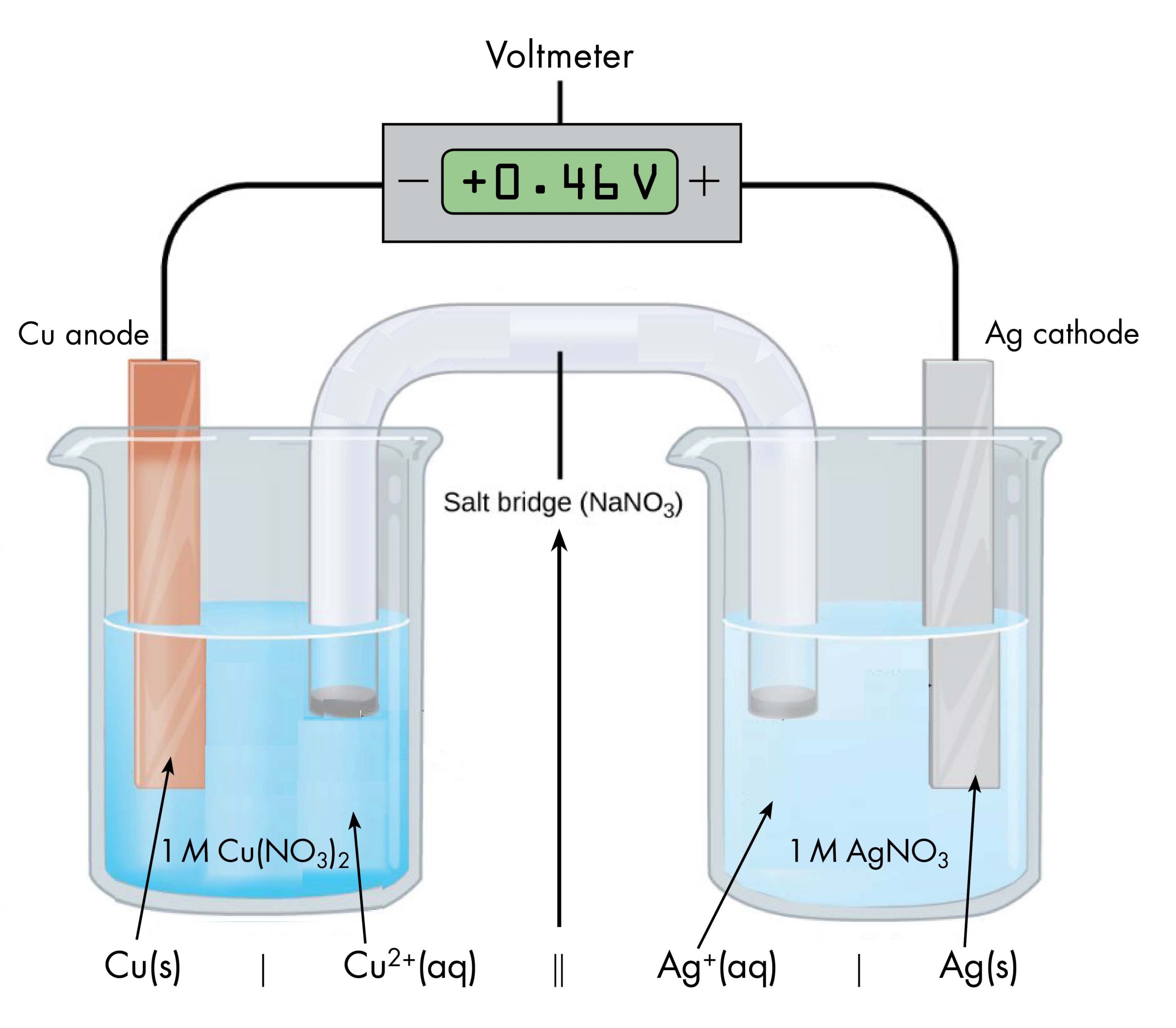
Note that spectator ions, such as NO3−, are not included in the cell notation, and if there are coefficients in a half-reaction, the coefficients are not included (that is, the coefficients of 2 in the silver half-reaction do not appear in the cell notation). When known, the initial concentrations of ions are usually included in the cell notation, so a more complete cell notation for the cell above is Cu(s) | Cu2+(aq, 1 M) || Ag+(aq, 1 M) | Ag(s).
Some redox reactions involve species that are poor conductors of electricity, such as gases or ionic solids. For such substances, an inert electrode that does not participate in the reactions is used. An example of such a voltaic cell is shown below.
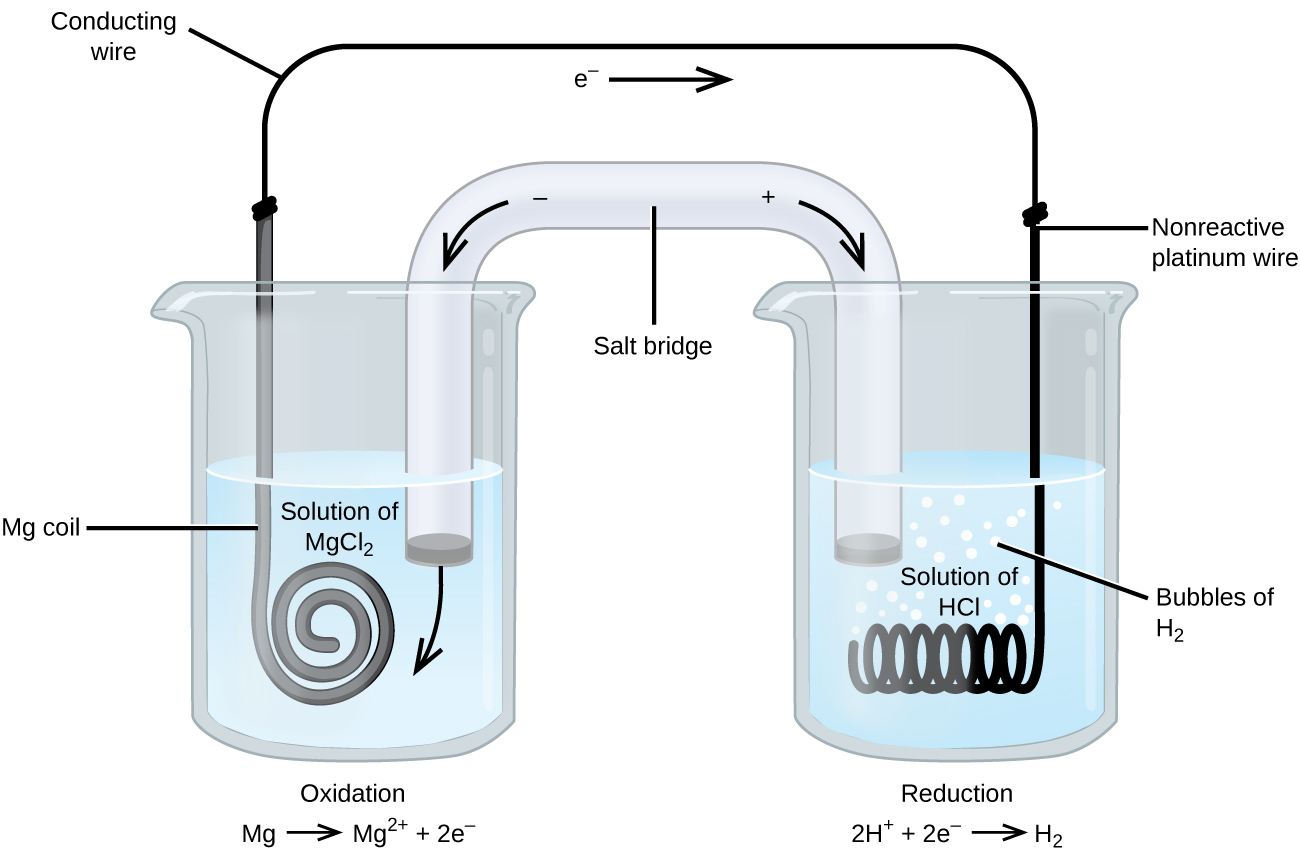
The redox reaction involved is:
| Oxidation (anode): | Mg(s) | ⟶ | Mg2+(aq) + 2e− |
| Reduction (cathode): | 2H+(aq) + 2e− | ⟶ | H2(g) |
| overall: | Mg(s) + 2H+(aq) | ⟶ | Mg2+(aq) + H2(g) |
This voltaic cell uses an inert platinum wire for the cathode, so the cell notation is:
The magnesium electrode is an active electrode because it participates in the redox reaction. Inert electrodes, like the platinum electrode, do not participate in the redox reaction but must be present so that there is a complete electrical circuit. Platinum and gold are among the least reactive metals so they are good choices for inert electrodes. Graphite, also inert to many chemical reactions, is another common option.
Activity: Half-reactions and Cell Notation
Consider a voltaic cell based on this spontaneous reaction:
5Fe2+(aq) + MnO4−(aq) + 8H+(aq) ⟶ 5Fe3+(aq) + Mn2+(aq) + 4H2O(ℓ)
Write the oxidation and reduction half-reactions and use cell notation to describe a voltaic cell based on this reaction. When the cell produces electric current, which reaction occurs at the anode? Which occurs at the cathode?
Write in your notebook, then left-click here for an explanation.
Assigning oxidation numbers to each element in each species in the overall redox reaction shows that Fe2+ undergoes oxidation when one electron is lost to form Fe3+, and MnO4− is reduced as it gains five electrons to form Mn2+.
Thus the balanced half-reactions are
| Oxidation: | 5 × (Fe2+(aq) | ⟶ | Fe3+(aq) + e−) |
| Reduction: | MnO4−(aq) + 8H+(aq) + 5e− | ⟶ | Mn2+(aq) + 4H2O(ℓ) |
When the cell generates electric current, the oxidation half-reaction occurs at the anode and the reduction half-reaction occurs at the cathode.
Cell notation lists the species in each half-cell, starting with the anode. The anode (and the cathode) half-reaction involves only ions in solution so there is no solid-phase metal involved; therefore it is necessary to use an inert electrode, such as platinum, to conduct electrons out of the anode half-cell. Additionally, no concentration information is provided. Hence, the cell notation is
Pt(s) | Fe2+(aq), Fe3+(aq) || MnO4−(aq), H+(aq), Mn2+(aq) | Pt(s)
Exercise: Cell Notation
A historically important voltaic cell is the Daniell cell, a variant of which, the gravity cell, was used to power telegraph communication in the late 19th century. In a Daniell cell, zinc reacts with copper(II) ions to generate electric current.
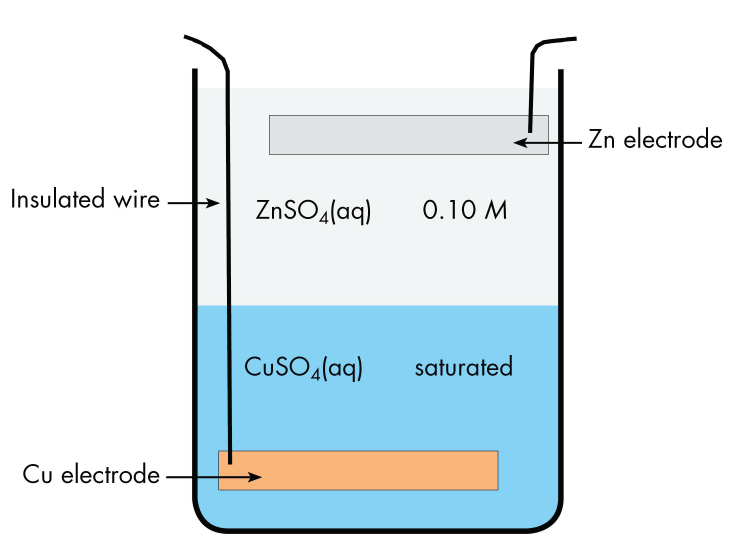
The cell has a copper electrode at the bottom of a jar with an insulated wire connecting it to the outside. The bottom half of the jar contains saturated copper(II) sulfate solution. The upper half of the jar contains dilute zinc sulfate solution, which is less dense than the copper(II) sulfate and can be carefully poured onto the copper(II) sulfate without mixing the solutions. At the top of the zinc sulfate solution is a zinc electrode, suspended from the rim of the jar. Current flows in a wire connecting the copper electrode to the zinc electrode. There is no salt bridge, but ions can move in either direction across the interface between the two solutions.
Write the cell notation for the gravity cell.
Write in your notebook, then left-click here for an explanation.
The cell notation is
Zn(s) | Zn2+(aq) | Cu2+(aq) | Cu(s)
According to the description of the gravity cell zinc reacts with copper(II) ions. Thus, Zn2+ ions and Cu(s) atoms must be produced. Therefore Zn must be oxidized and copper(II) ions must be reduced. The Zn half-cell is the anode and the copper(II) half-cell is the cathode, so the Zn half-cell is written first in the cell notation.
There is no double vertical line because there is no salt bridge. The single vertical line in the middle denotes the interface between the copper(II) sulfate solution below and the zinc sulfate solution above.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

