D15.6 Copolymers
Some of the most commercially important addition polymers are copolymers, made by polymerizing a mixture of two or more monomers. For example, styrene-butadiene rubber (SBR), which is a copolymer of 1,3-butadiene and styrene mixed in about a 3:1 ratio.
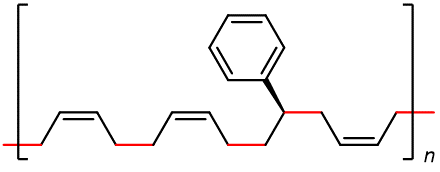
The properties of a copolymer are distinctive. For instance, the properties of SBR are different from a mixture containing polybutadiene and polystyrene, no matter what the ratio of the mixture is. This is because individual polymer strands in SBR are different from individual strands in polybutadiene and polystyrene.
SBR was developed in the U.S. during World War II when important supplies of natural rubber were cut off. It is more resistant to abrasion and oxidation than natural rubber and can also be vulcanized. More than 40% of the synthetic rubber production is SBR, which is used in tire production. Several other types of rubber are copolymers, such as butyl rubber, which is copolymerized from 2-methylpropene (H2C=C(CH3)2) and a small percentage of isoprene.
Exercise: Monomer(s) from Polymer Structure
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

