Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 15 Pre-Class Podia Activity
Viscosity is a term we use to describe the “thickness” of different liquids. For example, we say that honey is more viscous than water. When we say that, we mean that water is much easier to stir or to pour than honey. Equimolar solutions of linear alkanes, CnH2n+2, become increasingly viscous with increasing molecular weight (i.e., with increasing number of carbon atoms, n). However, as shown in the plot at right, viscosity does not increase with molecular weight in a linear way.
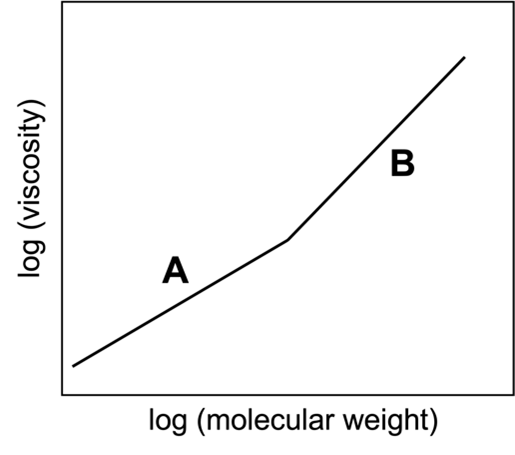
- Construct a picture that illustrates why a solution of longer molecules would be more resistant to flow than a solution of shorter molecules. Use this picture to explain the linear increase in viscosity with solutions of lower molecular weight alkanes (section A of the graph above).
- Why does the graph “bend” to give rise to a steeper slope (section B of the graph above) at a certain molecular weight? What might be happening in solutions of VERY long molecules that your model from part (a) does not account for? How does this extra factor contribute to increased viscosity?
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

