Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 20 Pre-Class Podia Activity
Consider the isomerization of trans-2-butene to cis-2-butene as shown below.
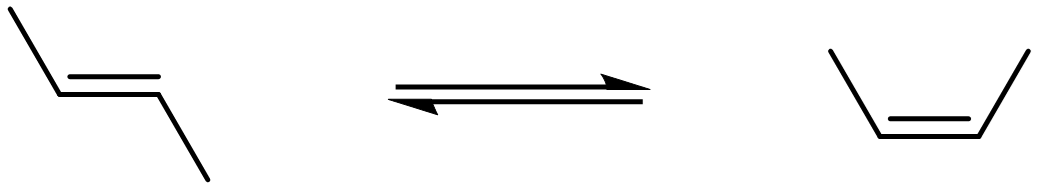
- The equilibrium constant (KP) for this isomerization at 1000 K is 0.67. Sketch five of each molecule in an imaginary flask—this will be our starting condition at 1000 K. Then, sketch how the reaction mixture would appear when equilibrium has been attained.
- At 400 K, the equilibrium constant (KP) for this isomerization is 0.48. If a flask was filled with cis-2-butene at a partial pressure of 1 bar, sketch how the concentration of trans-2-butene would change over time until equilibrium is reached. (Be as accurate in beginning and ending partial pressures of trans-2-butene as possible.)
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

