Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 24 Pre-Class Podia Activity
In lab this week, you synthesized acetaminophen from 4-aminophenol and acetic acid. (Lab director’s note: yes, it was acetic anhydride used in this reaction in lab this week. We’ve chosen a simpler model for this question in order to better focus on some important themes.)
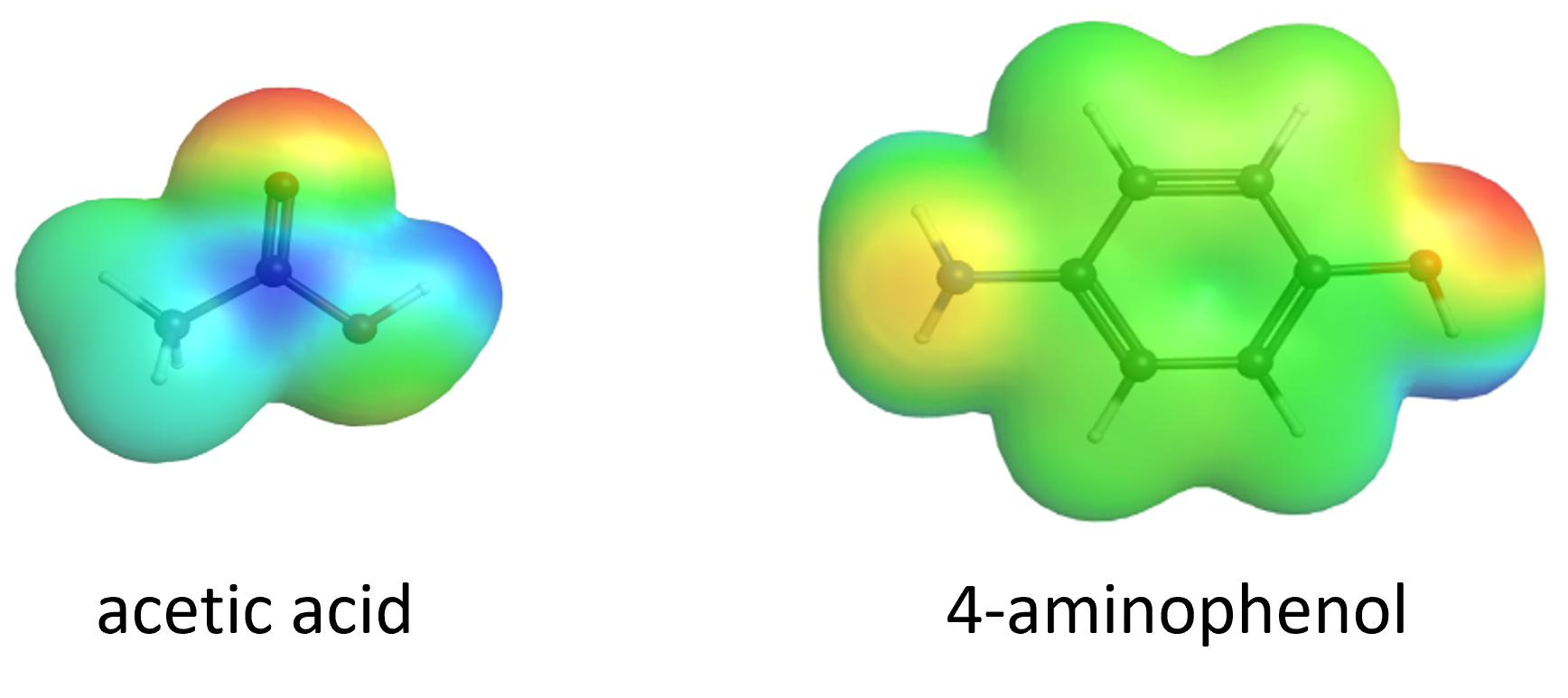
Reactant molecules often collide in a chemical reaction according to electrostatic attractions—i.e., the reactant molecules collide in a way such that a center of negative charge on one molecule interacts with a center of positive charge on another molecule.
Illustrate how acetic acid and 4-aminophenol must collide in a very specific orientation to form acetaminophen. Provide evidence for your claim using the electrostatic potential maps of the two reactant molecules above (blue regions illustrate positive charge while red regions illustrate negative charge). Connect your claim and evidence using reasoning related to Coulomb’s law.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

