Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 25 Pre-Class Podia Activity
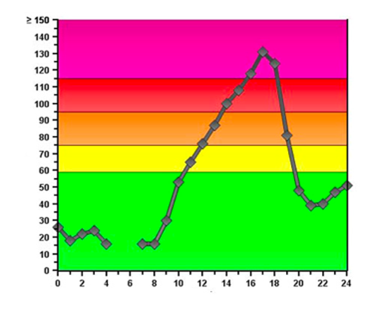 Refer to the plot of ozone concentration in parts per billion versus hour of day at right. Ozone formation is faster during the day due to the action of sunlight on NO2 molecules. Thus, O3(g) accumulates in the troposphere and its concentration peaks sometime in the afternoon. During the night, however, ozone reacts with NO(g) according to the following reaction:
Refer to the plot of ozone concentration in parts per billion versus hour of day at right. Ozone formation is faster during the day due to the action of sunlight on NO2 molecules. Thus, O3(g) accumulates in the troposphere and its concentration peaks sometime in the afternoon. During the night, however, ozone reacts with NO(g) according to the following reaction:
NO(g) + O3(g) ⇌ NO2(g) + O2(g)
This process has an activation energy of 12.5 kJ/mol, and a ΔrG° of –198.2 kJ/mol.
- Build a reaction progress diagram that represents the Gibbs energy change as reactants transform into products. Indicate on the diagram the activation energy and the Gibbs energy change for this process.
- Which process associated with this reaction (the forward or the reverse, as written) will have the larger rate constant? What feature of your reaction progress diagram was important for making this claim?
- Estimate the equilibrium constant K for this reaction at 298 K using the activation energies of the forward and reverse processes.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

