Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 29 Pre-Class Podia Activity
The decomposition of amino acids (according to the reaction shown below with alanine) is thermodynamically favored under standard conditions. However, this process may take so long that amino acids may combine to form proteins before decomposing.
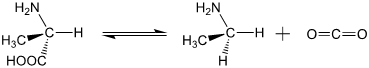
The data tables below show the decomposition in the concentration of alanine as a function of time for alanine decomposition at two different temperatures: 150 °C and 200 °C.
| 150 °C | 200 °C | ||
| time (years) | [Ala] (mM) | time (days) | [Ala] (mM) |
| 0 | 1.000 | 0 | 1.000 |
| 1 | 0.933 | 1 | 0.962 |
| 2 | 0.871 | 2 | 0.925 |
| 3 | 0.812 | 3 | 0.889 |
| 4 | 0.758 | 4 | 0.855 |
| 5 | 0.707 | 5 | 0.823 |
| 6 | 0.660 | 6 | 0.791 |
| 7 | 0.616 | 7 | 0.761 |
| 8 | 0.574 | 8 | 0.732 |
- Calculate the average rate of reaction between years 2 and 3, and between years 6 and 7, for T = 150 °C
- Discuss how you would go about estimating the instantaneous rate of the reaction at year 4.
- How does the rate of reaction change as the concentration of alanine in the system decreases? How would you explain this result?
- How does the rate of reaction change when raising the temperature? How would you explain this result?
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

