Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 31 Pre-Class Podia Activity
The following reaction progress diagram represents the synthesis of NH3 from N2 and H2 over the surface of a ruthenium (Ru) catalyst. The symbol * denotes an empty site on the catalyst and X* (e.g., N2* or N*) represents an adsorbed species.
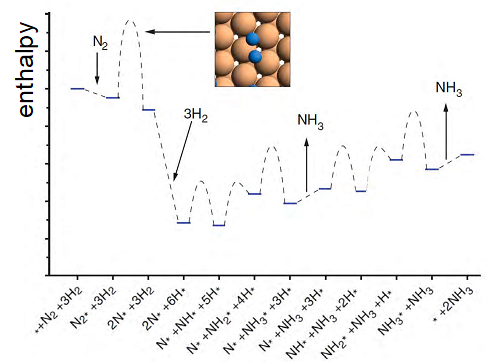
- Describe the sequence of elementary steps that take place.
- Identify all the intermediate species.
- Discuss how the catalyst provides reaction path with a lower effective activation energy.
- What processes decrease the enthalpy of the reacting species?
- Identify the rate-limiting step of the process.
- Assuming that all of the steps that only involve adsorption or desorption of species are fast and achieve equilibrium, derive the overall rate law for ammonia synthesis in the presence of a catalyst. Use the following notation to represent the concentration of the relevant species: [N2], [H2], [*] (this symbol represents the concentration of empty adsorption sites on the catalyst).
- Based on your results, discuss how doubling the partial pressure of N2 and H2 should affect the rate of reaction.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

