Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 38 Pre-Class Podia Activity
One of the most important natural buffers includes the acid-base pair H2PO4–/HPO42– (pKa = 7.1). The graph included below shows how the pH of this phosphate buffer changes with the percentage of the conjugate base (HPO42–) in solution.
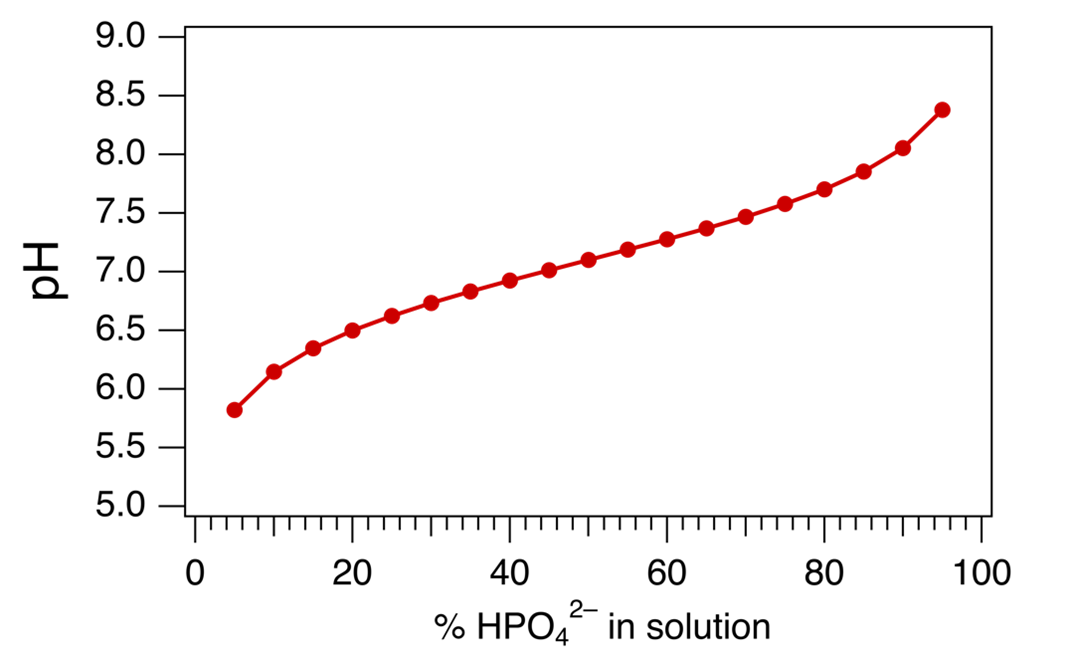
- Consider creating a H2PO4–/HPO42– buffer that experiences the least change in pH when acid or base is added. Use the graph to decide which of the following buffer solutions would meet this criterion:
- 80% H2PO4– and 20% HPO42–
- 60% H2PO4– and 40% HPO42–
- 50% H2PO4– and 50% HPO42–
Explain briefly how you used the graph to make your choice.
- Discuss how the graph would be different for another buffer pair, such as H3PO4/H2PO4– (pKa = 2.16).
- What ratio of [H2PO4–]/[H3PO4] would be best to use to prepare a buffer with the H3PO4/H2PO4– pair?
- Discuss the implications of your previous responses for the preparation of buffer solutions that can best resist changes in pH when other acids or bases are added to them.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

