Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 41 Pre-Class Podia Activity
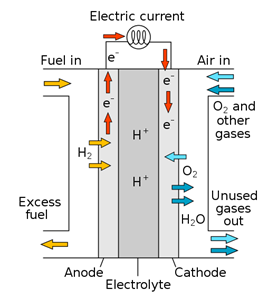 Fuel cells are electrochemical devices in which reactants are continuously supplied to sustain the redox reaction. In a hydrogen fuel cell, for example, hydrogen gas and oxygen gas from air are used to produce energy. The overall reaction that takes place in the hydrogen fuel cell can be represented as:
Fuel cells are electrochemical devices in which reactants are continuously supplied to sustain the redox reaction. In a hydrogen fuel cell, for example, hydrogen gas and oxygen gas from air are used to produce energy. The overall reaction that takes place in the hydrogen fuel cell can be represented as:
H2(g) + ½ O2(g) ⇌ H2O(ℓ)
- Determine the oxidation number of each atom in the reactants and products and identify the oxidized and reduced atoms in this process.
- Determine the number of electrons exchanged per mole of H2 consumed.
- The table below lists relevant thermodynamic information for reactants and products in a hydrogen fuel cell.
ΔfH° (kJ/mol) S° (J/mol·K) H2(g) 0 130.6 O2(g) 0 205.0 H2O(ℓ) -285.8 69.95 Compute ΔrG° at 25 °C and 70 °C (typical temperatures at which a hydrogen fuel cell operates).
- Estimate the value of E°cell at 25 °C and 70 °C. Using enthalpy and entropy ideas, build a plausible explanation for the change of E°cell with temperature.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

