D25.2 Effect of Temperature on Equilibrium
Recall that:
Therefore:
This equation can be used to calculate K° at different temperatures, if we assume that ΔrH° and ΔrS° for a reaction have the same values at all temperatures. This is a good, but not perfect, assumption. It is not a good assumption if there is a phase change for a reactant or a product within the temperature range of interest.
Dividing both sides of the equation by -RT gives:
| lnK° | = | + | ||
| y | = | mx | + | b |
A plot of lnK° vs. ![]() is called a van’t Hoff plot. The graph has a slope of
is called a van’t Hoff plot. The graph has a slope of ![]() . Therefore, if the concentrations of reactants and products are measured at various temperatures so that K° can be calculated at each temperature, the reaction’s ΔrH° can be obtained from a van’t Hoff plot.
. Therefore, if the concentrations of reactants and products are measured at various temperatures so that K° can be calculated at each temperature, the reaction’s ΔrH° can be obtained from a van’t Hoff plot.
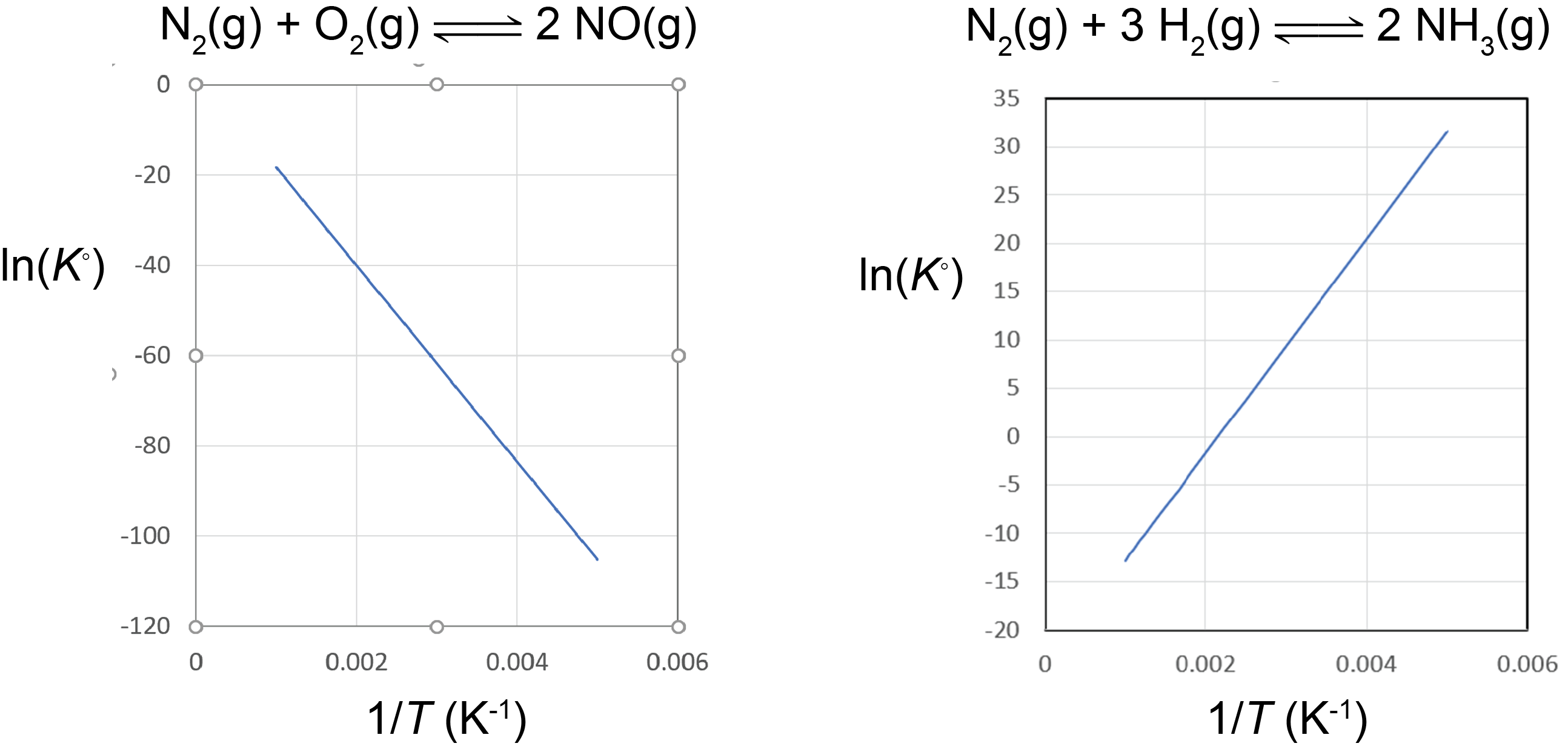
Based on the equation for the van’t Hoff plot, an exothermic reaction (ΔrH° < 0) has K° decreasing with increasing temperature, and an endothermic reaction (ΔrH° > 0) has K° increasing with increasing temperature. The magnitude of ΔrH° will dictate how rapidly K° changes as a function of temperature.
For example, suppose that K°1 and K°2 are the equilibrium constants for a reaction at temperatures T1 and T2, respectively:
Subtracting the two equations yields:
![Rendered by QuickLaTeX.com \begin{array}{rcl}\text{ln}K_2^{\circ}\;-\;\text{ln}K_1^{\circ} &=& \left(-\dfrac{{\Delta}_{\text{r}}H^{\circ}}{R}\left(\dfrac{1}{T_2}\right)\;+\;\dfrac{{\Delta}_{\text{r}}S^{\circ}}{R}\right) - \left(-\dfrac{{\Delta}_{\text{r}}H^{\circ}}{R}\left(\dfrac{1}{T_1}\right)\;+\;\dfrac{{\Delta}_{\text{r}}S^{\circ}}{R}\right)\\[1em] \text{ln}\left(\dfrac{K_2^{\circ}}{K_1^{\circ}}\right) &=& -\dfrac{{\Delta}_{\text{r}}H^{\circ}}{R}\left(\dfrac{1}{T_2}\;-\;\dfrac{1}{T_1}\right)\\[1em] \text{ln}\left(\dfrac{K_2^{\circ}}{K_1^{\circ}}\right) &=& \dfrac{{\Delta}_{\text{r}}H^{\circ}}{R}\left(\dfrac{1}{T_1}\;-\;\dfrac{1}{T_2}\right) \end{array}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-01d6ef5b672425928aa18d3d5dc9face_l3.png)
Thus calculating ΔrH° from tabulated ΔfH° values and measuring the equilibrium constant at one temperature would allow us to calculate the equilibrium constant at any other temperature (assuming that ΔrH° and ΔrS° are independent of temperature).
Using this equation, you can also estimate ΔrH° without constructing a van’t Hoff plot if K° was determined at only two temperatures (but the result will not be as accurate as a van’t Hoff plot constructed from numerous data points).
Left-click here for different forms of the equation
You can multiply both side of the equation by -1 and obtain:
![]()
![]()
Whichever form of the equation you use, keep careful track of the subscripts on the variables K1°, T1, K2°, and T2.
Exercise: Temperature Dependence of the Equilibrium Constant
Left-click here to read more about Van’t Hoff plots and ΔrG° vs. T relationships
You may have noticed that when a reaction is product-favored or reactant-favored at all temperatures, it appears that ΔrG° and K° have opposite trends with respect to changes in temperature. This is a rather subtle and complex issue, and one which we will not delve deeply into in this course. But we can unravel this a little bit by considering what we already know.
The relationship between ΔrG° and K° has a temperature dependence:
ΔrG° = −RT(lnK°)
While ΔrG° itself has a temperature dependence:
ΔrG° = ΔrH° – TΔrS°
and K° itself also has a temperature dependence:
![]()
Therefore, temperature dependence of ΔrG° is informed by the rising impact of the entropy term (ΔrS°). In contrast, temperature dependence of K° is informed by the shrinking impact of the enthalpy term (ΔrH°). Usually, the two impacts point in the same direction, but this is not necessarily the case in the always product-favored or always reactant-favored situations.
Keep in mind that these temperature relationships we are discussing are simplified: we are not accounting for the possible temperature dependence of ΔrH° and ΔrS° terms.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

