D30.5 Homogeneous Catalysis
Reactions that are facilitated by catalysts can be divided into two classes: homogeneous catalysis and heterogeneous catalysis. A homogeneous catalyst is present in the same phase as the reactants. We have already discussed several examples where catalyst and reactants are all in the same phase.
Another example is acid-catalyzed decomposition of methyl acetate to form acetic acid and methanol, a type of ester hydrolysis. (Hydrolysis is the reverse reaction of a condensation reaction that produces water as the byproduct.) This is a homogeneous catalytic reaction because reactants, products, and catalyst are all in aqueous solution.
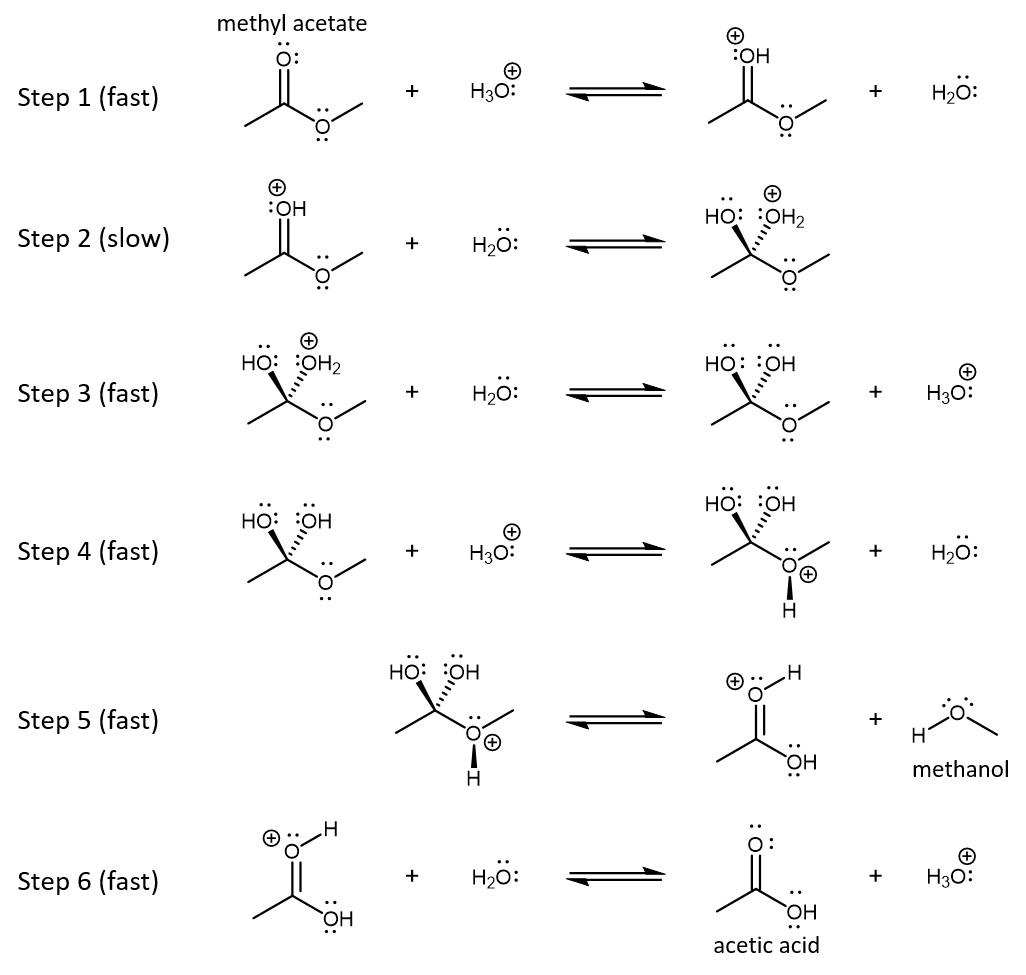
The reaction mechanism is shown below. Each step in the mechanism is an acid-base reaction, a type of reaction that we will learn about in more detail in the next unit.
Activity: Analyzing a Reaction Mechanism
Consider the mechanism for acid-catalyzed hydrolysis of methyl acetate in aqueous solution (Figure: Hydrolysis). In your notebook,
- Write the overall reaction based on the given mechanism.
- Explain clearly how the mechanism supports the description, “acid-catalyzed”.
- Study each step in the mechanism from the perspective of chemical bonding. Try to identify the movements of electrons and the changes in positions of bonds in each reaction step. Write a description of electron movements for each step.
Write in your notebook, then left-click here for an explanation.
- The overall reaction is
CH3COOCH3(aq) + H2O(ℓ)
![Rendered by QuickLaTeX.com \xrightleftharpoons[]{H_3O^+}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-98f77463f4ae23bcc36bd3fa8ca301c6_l3.png) CH3COOH(aq) + CH3OH(aq)
CH3COOH(aq) + CH3OH(aq)(A catalyst is typically written over the reaction arrow to indicate its presence in the reaction)
- Acid, H3O+, is a reactant in the first step of the mechanism and is reformed as a product in the third step in the mechanism. Formation of the intermediate in step 1 results in a slow step (step 2) that has lower activation energy than would direct reaction of H2O with a methyl acetate molecule with no positive charge. Thus, the acid increases the rate of reaction. (H3O+ is also a reactant in step 4 and is regenerated in the last step, but these steps come after the rate-determining step and do not affect the rate.)
H3O+ is not consumed by the reaction (and does not show up in the net overall reaction). - Here is an analysis of bonding in each step in the mechanism.
Step 1: One of the lone pairs on the carbonyl oxygen in methy acetate forms a new bond to one of the hydrogens in H3O+. The two electrons that formed the O-H bond in H3O+ become a lone pair on O in water.
Step 2: One pair of electrons in the C=O double bond transfers to the O atom, becoming a lone pair. A lone pair of electrons in the water molecule forms a new single bond to the carbonyl carbon atom.
Step 3: One of the lone pairs in a water molecule forms a bond with one of the hydrogens in the −C−OH2+ group. The water molecule becomes an H3O+ ion. The pair of electrons that originally constituted the O-H bond becomes a lone pair on the O atom in what had been the −C−OH2+ group.
Step 4: One of the lone pairs in the C-O-C group forms a bond with one of the hydrogens in H3O+. The two electrons that formed the O-H bond in H3O+ become a lone pair on O in water.
Step 5: The C-O bond to the O atom with positive formal charge breaks and the pair of electrons that were in that bond stays on the oxygen atom; a methanol molecule is formed (leaves the intermediate) by this bond breaking. A pair of electrons on O in one of the -OH groups moves between the O and C atom, reforming the double bond to the carbonyl carbon atom; this produces an acetic acid molecule with one extra proton on the carbonyl oxygen.
Step 6: One of the lone pairs in a water molecule forms a bond with the hydrogen on the carbonyl oxygen, forming a new O-H bond in H3O+. The two electrons that fromed the bond between the carbonyl oxygen and the hydrogen atom become a lone pair on the carbonyl oxygen. An uncharged acetic acid product and a H3O+ ion are formed.
Using the equilibrium approximation, derive the rate law for this reaction based on the given mechanism.
Write in your notebook, then left-click here for an explanation.
The rate-determining step in this reaction is step 2. The rate law for step 2 is:
rate2 = k2[methylacetateH+]
Assuming that step 1 is at equilibrium, we have:
k1[methylacetate][H3O+] = k−1[methylacetateH+]
[methylacetateH+] = ![]() [methylacetate][H3O+]
[methylacetate][H3O+]
Substituting into the rate law for step 2, approximating that the rate law for step 2 is the rate law for the overall reaction, we have:
rate = ![]() [methylacetate][H3O+] = k′[methylacetate][H3O+]
[methylacetate][H3O+] = k′[methylacetate][H3O+]
This reaction is first-order with respect to the concentration of methyl acetate and first-order with respect to the concentration of the acid catalyst.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

