D14.5 Intermolecular Forces: Hydrogen Bonding
Applying Core Ideas: Comparing Propane, Dimethyl Ether, and Ethanol
The additional IMF that exists between ethanol molecules, but does not exist between propane molecules or between dimethyl ether molecules, is called hydrogen bonding. It is the interaction between an X–H covalent bond (X denotes a highly electronegative atom) and the lone pair on an electron rich atom, Z. The hydrogen bonding interaction can be designated as X-H···Z (the “···” is the hydrogen bond itself).
Strong hydrogen bonding occurs between F-H, O-H, or N-H bond and an electron lone pair on another F, O, or N atom. F, O, and N, are among the most electronegative elements in the periodic table, a characteristic necessary for strong hydrogen bonding.
A hydrogen bond has about 5-10% the strength of a typical covalent bond. Part of this strength comes from dipole-dipole interactions, but more important is partial electron sharing that resembles the formation of a covalent bond. This is illustrated in the figure below, which shows the hydrogen bond interaction between two water molecules. The left water molecule provides the O-H bond (we will denote this as OL-H); the right water molecule provides the O atom with the lone pair (we will denote this as OR).
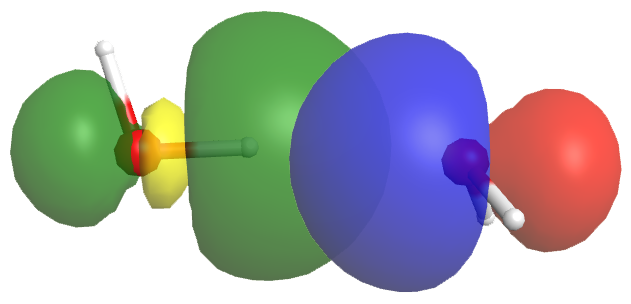
The empty OL-H antibonding σ* orbital (yellow/green) overlaps with the lone pair orbital of the OR atom (blue/red). This overlap allows some electron density to be shared between the two water molecules, thus forming a hydrogen bond. If more electron density were to move from the OR lone pair to the OL-H σ* orbital, the antibonding orbital would be filled, the OL-H bond would break, and a chemical reaction would have occurred—a new OR-H bond would form, resulting in the right water molecule becoming H3O+:
Thus, forming a hydrogen bond resembles the beginning of the formation of a covalent bond.
Activity: IMFs between Water Molecules and between Hydrogen Fluoride Molecules
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

