D20.4 Le Châtelier’s Principle: Change in Concentration
Using an equilibrium constant and ICE table, we can calculate equilibrium concentrations. Using Q and K we can analyze which direction a system that is not in equilibrium reacts to establish equilibrium. Often, however, we do not know exact concentrations or partial pressures of reactants and products. In such a case Le Châtelier’s principle enables prediction of which direction an equilibrium will shift when conditions change.
Le Châtelier’s principle states that when a chemical system is at equilibrium and conditions are changed so that the reaction is no longer at equilibrium, the chemical system reacts to achieve new equilibrium concentrations or partial pressures in a way that partially counteracts the change in conditions.
For a system at equilibrium at constant temperature, if the concentration of a reactant or a product is changed, therefore changing Q, the system is no longer at equilibrium because Q ≠ K. The concentrations of all reaction species will then undergo additional changes until the system reaches a new equilibrium with a different set of equilibrium concentrations. Le Chatelier’s principle predicts that the reaction will proceed in a direction (forward or reverse) that would partially counteract the initial concentration change.
For example, consider a generic chemical reaction:
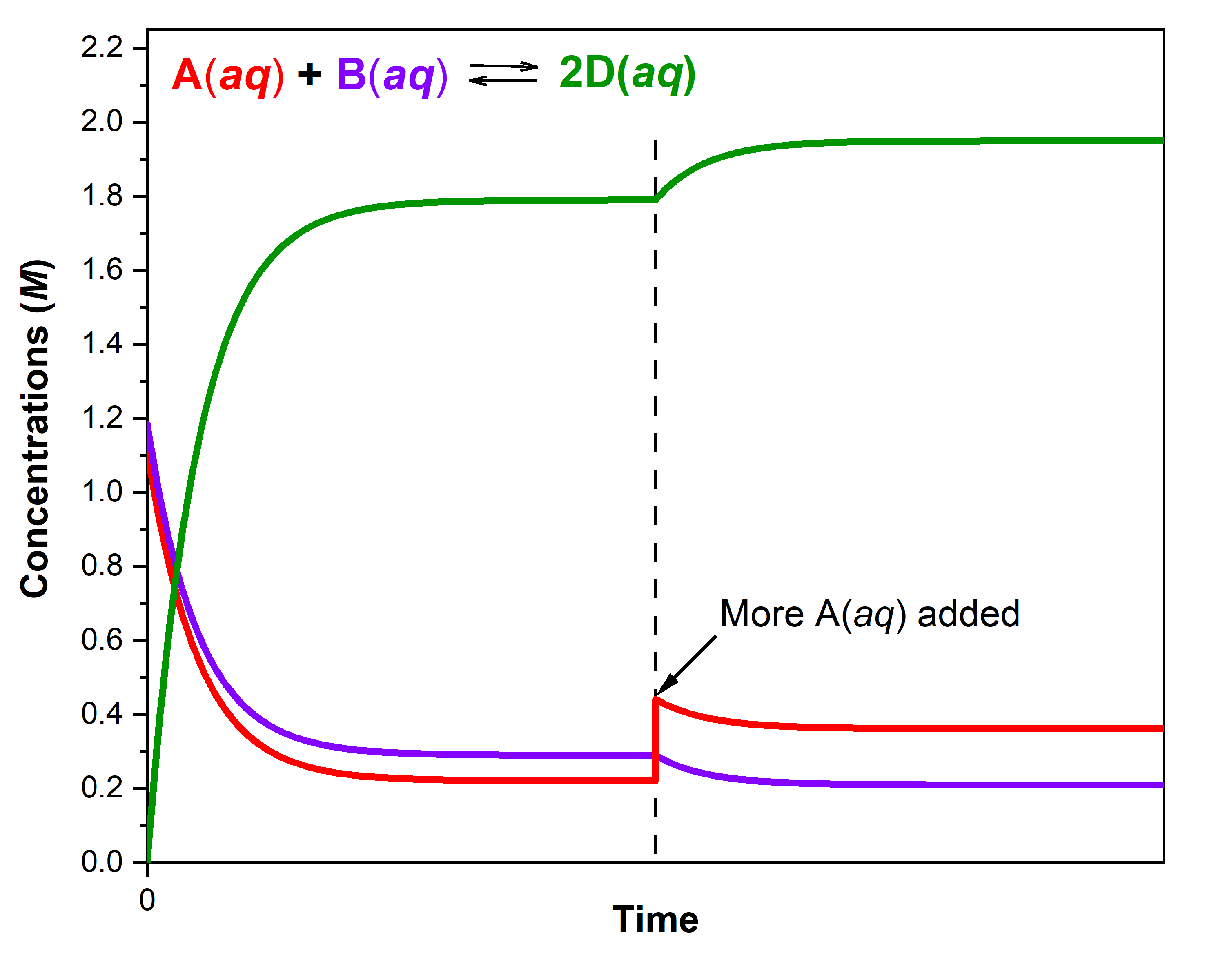
A solution at 45 °C with with [A] = 0.221 M, [B] = 0.290 M, and [D] = 1.790 M is at equilibrium. For this mixture, Q = K = 50.
If, at time = t, additional A(aq) is introduced into the solution quickly such that [A] doubles before it begins to react (that is, the new [A]t = 0.442 M), Q is now ½ of K:
The reaction will proceed in the forward direction (towards products) to reach a new equilibrium. You can use an ICE table and calculate that the new equilibrium concentrations are [A] = 0.362 M, [B] = 0.210 M, and [D] = 1.950 M.
Notice that [A]new equilibrium = 0.362 M is less than the doubled concentration ([A]t = 0.442 M) but more than [A]first equilibrium = 0.221 M. The reaction has proceeded in a direction that partially counteracts the change in concentration of A. Because of this, the concentration of the other reactant decreases and the concentration of the product increases. To verify that these new concentrations are equilibrium concentrations, calculate Q:
The figure below illustrates graphically the effect of adding A(aq) to the reaction that was at equilibrium.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

