D20.5 Le Châtelier’s Principle: Change in Pressure or Volume
Changes in pressure have a measurable effect on equilibrium in systems involving gases if the chemical reaction produces a change in the total number of gas molecules (that is, if the number of gas molecules on the reactant side differs from that on the product side). The overall change in pressure must affect partial pressures of reactants and/or products—adding an inert gas that is not a reactant or a product changes the total pressure but not the partial pressures of the gases in the equilibrium constant expression, and therefore does not perturb the equilibrium.
Consider what happens when the volume decreases for this equilibrium:
Decreasing the volume increases the total pressure and increases the partial pressure of each gas. The reaction will proceed to partially counteract the increase in pressure. Formation of additional NO2 decreases the total number of gaseous molecules in the system because each time two molecules of NO2 form, a total of three molecules of NO and O2 react away. This reduces the total pressure exerted by the system and partially counteracts the increase in pressure. Le Chatelier’s principle predicts that the equilibrium shifts to the right, toward products.
We can also look at this by considering Q. Reducing the system volume increases the partial pressure of all gaseous species. If the volume is reduced by half, then each partial pressure becomes twice what it was for the initial equilibrium:
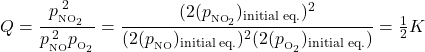
Because Q < K, the reaction proceeds toward the product side (forming additional NO2) to re-establish equilibrium.
Now consider this reaction:
Because there is no change in the total number of gaseous molecules in the system during reaction, a change in pressure does not shift the equilibrium towards either reactant or product side.
For reactions in solution, changing the solution volume changes the concentrations of all reaction species. Therefore, when the solution is diluted (by adding solvent), the reaction proceeds toward the side with more solute particles to establish a new equilibrium, partially compensating for the dilution of total concentration. If solvent is removed (by evaporation, for example) so that all solute concentrations increase, the reaction proceeds toward the side with fewer solute particles, decreasing the total concentration of solute particles.
For example, when enough water is added to this equilibrium to double the solution volume:
the concentration of each solute is halved compared to the initial equilibrium concentration. Hence:
![Rendered by QuickLaTeX.com Q = \dfrac{\frac{1}{2}[\text{C}_2\text{H}_2\text{Br}_4]_{\text{initial eq.}}}{\left(\frac{1}{2}[\text{C}_2\text{H}_2]_{\text{initial eq.}}\right)\left(\frac{1}{2}[\text{Br}_2]_{\text{initial eq.}}\right)^2} = 4K](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-5c48703dd99d76788450983cdb68199f_l3.png)
Because Q > K the reaction proceeds toward the reactant side (the side with more solute particles) as equilibrium is re-established.
Exercise: Using Le Chatelier’s Principle at Constant Temperature
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

