D6.3 MO Electron Configuration and Bond Order
Just as an atom has an electron configuration, so does a molecule. The molecular ground-state electron configuration can be obtained by filling electrons into MOs whose relative energies are shown in diagrams such as the one shown in Figure: MO Energy Levels, following rules similar to those for atomic electron configurations:
- Sum all the electrons from all the atoms that make up the molecule.
- Each MO can hold at most two electrons. (Two electrons occupying the same orbital must have opposite spin.)
- In the MO diagram the MOs are arranged in order of increasing energy.
- Add electrons to the lowest-energy MO first and then to successively higher-energy MOs (Aufbau principle).
- If two or more MOs have the same energy (are degenerate), apply Hund’s rule: assign one electron to each degenerate MO before pairing any electrons.
For the H2 molecule: there is one electron from each H atom, so there are two electrons in H2. There is only one lowest-energy MO, σ1s, therefore the two electrons, with opposite spin, will fill it. The ground state electron configuration of H2 is (σ1s)2.
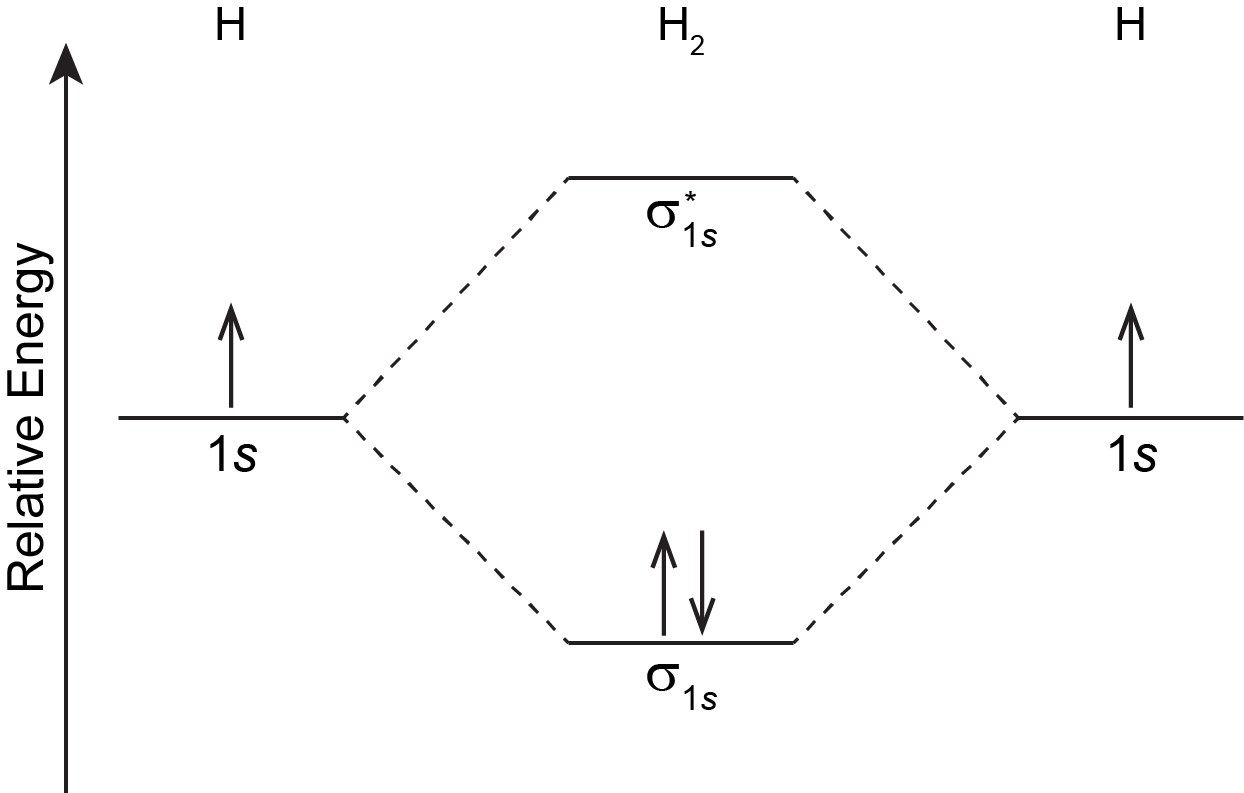
In Figure: MO Energy Levels in the previous section, and by convention in general, phases for occupied MOs are indicated by blue and red (when needed), while phases for unoccupied MOs are indicated by green and yellow. That is, σ1s is blue because it has two electrons and is all the same phase; σ*1s is green and yellow because there are no electrons in this MO and one part is opposite phase from the other.
Activity: Molecular Orbitals and Electromagnetic Radiation
Suppose that a photon interacts with a ground-state hydrogen molecule. Based on what you have already learned, propose at least two hypotheses about what might happen. Write each hypothesis in your notebook.
After writing in your notebook, click to see each hypothesis below. (There may be more than just these hypotheses.)
Hypothesis 1
Hypothesis 2
Hypothesis 3
Consider a situation in which a hydrogen molecule might emit a photon. Describe how you can use the wavelength of the emitted photon to learn something about the energy levels of the H2 molecule. Write your response in your course notebook.
Write in your notebook, then left-click here for an explanation.
The two electrons in the σ1s MO, which are shared between the two H atoms, form a covalent bond. The configuration (σ1s)2 indicates a single, sigma covalent bond. In general, a covalent bond involves overlap of two or more AOs that leads to increased electron densities close to atomic nuclei so that the electrons’ energies are lowered. Electrons are shared between pairs of atomic nuclei, or more widely among several nuclei within a molecule.
Bond Order
In some cases more than two electrons can be shared between two nuclei. This gives rise to the idea of bond order, which is the number of shared electron pairs between two atoms. Using a MO diagram, bond order can be defined as:
where nbonding e‾ is the number of electrons in bonding MOs and nantibonding e‾ is the number of electrons in antibonding MOs. There is a factor ½ because two shared electrons make one bond. In general, a greater number of shared electrons means a stronger bond, so the larger the bond order is, the stronger the bond is.
Exercise: Bond Order
Activity: Molecular Orbitals for He2
Draw a MO energy level diagram for He2, filling all electrons into MOs. Write the MO electron configuration for He2. Based on the diagram and electron configuration, calculate the bond order for He2.
There are only London dispersion forces between He atoms. The atoms do not form a covalent chemical bond. Write a scientific explanation for this fact. Use the MO energy level diagram and electron configuration as the basis for your explanation.
Draw and write in your notebook, then left-click here for an explanation.
The MO energy level diagram for He2 is shown below. In the diagram there are two electrons in the sigma bonding MO and two electrons in the sigma antibonding MO. The MO electron configuration is (σ1s)2(σ∗1s)2. The bond order is zero because there are two bonding electrons and two antibonding electrons.
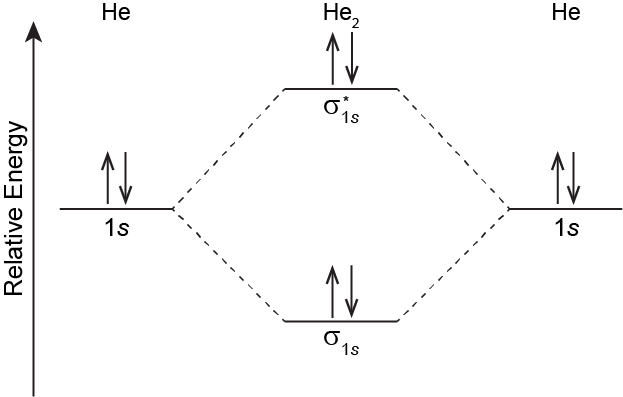
According to the MO energy level diagram, the energy of the sigma bonding MO is below the energy of the He 1s orbitals in the separated atoms. The energy of the sigma antibonding MO is higher than the energy of the He 1s orbitals by a quantity approximately equal to the energy lowering of the bonding MO. Thus, if a He2 molecule were to form, the total energy would be the same as for the two separated He atoms. Because there is no energy lowering for He2 relative to 2 He, no covalent bond forms: the bond order is zero. With no covalent bonding, only weak LDFs attract two He atoms.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

