D11.6 Molecules with More Than One Central Atom
Larger molecules have more than one “central” atom with several other atoms bonded to it. The arrangement of bonds for each central atom can be predicted as described in the preceding sections. The way these local structures are oriented with respect to each other influences the overall molecular shape.
Activity: Molecules with Several Central Atoms
Consider the Lewis structure below, where each central atom is numbered. The structure is not drawn with wedge-dash notation.
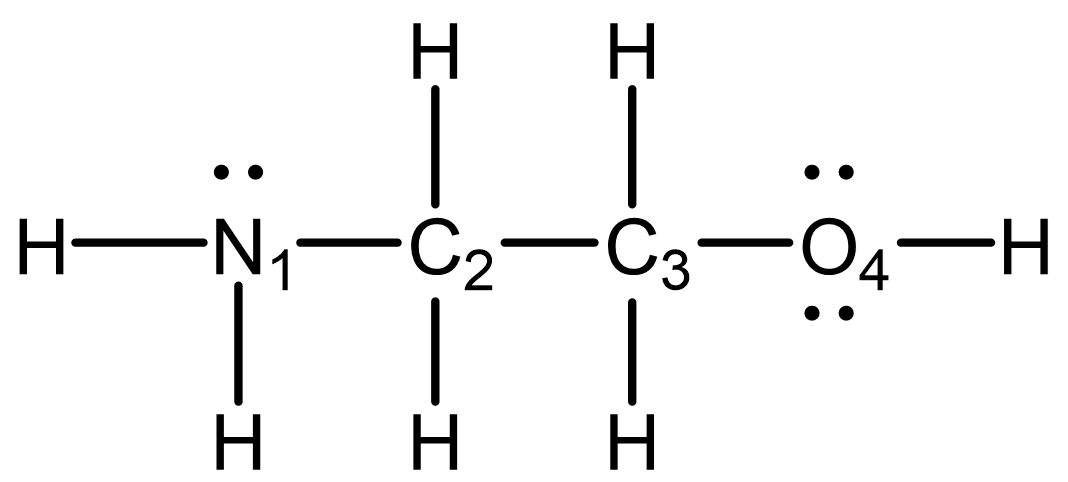
In your notebook:
- Identify the hybridization for each atom (except H atoms) in the structure.
- Determine the arrangement of bonds around each atom and describe the local structure.
- Indicate whether the structure is three dimensional.
Write in your notebook, then left-click here for an explanation.
- N1 is sp3 hybridized
C2 is sp3 hybridized
C3 is sp3 hybridized
O4 is sp2 hybridized - N1 has three bonds arranged as a trigonal pyramid.
C2 has four bonds pointing to the corners of a tetrahedron.
C3 has four bonds pointing to the corners of a tetrahedron.
O4 has two bonds in an angular or bent configuration (nonlinear). - Three of the four central atoms are three-dimensional, so the molecule cannot be planar or linear. The molecule is three-dimensional.
Exercise: Identifying Hybridization
Activity: Predicting Structure of a Molecule with Several Central Atoms
The simple Lewis structure for acetone is shown below. Predict the hybridization and local geometry for all the non-hydrogen atoms. Draw the molecule with correct 3D geometry using the wedge-dash notation.
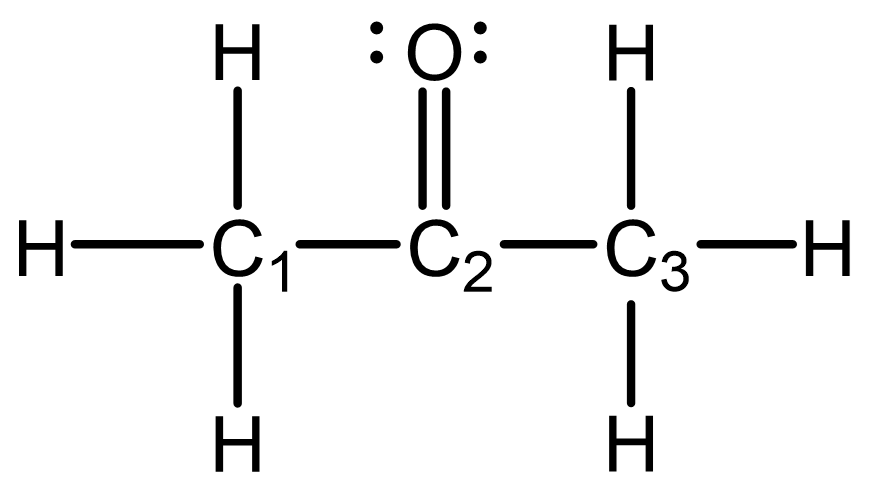
Draw in your notebook, then left-click here for an explanation.
The hybridization and local geometry for each non-hydrogen atom is:
- C1: sp3; tetrahedral
- C2: sp2; trigonal planar
- C3: sp3; tetrahedral
- O: sp; linear
The Lewis structure, drawn with wedge-dash notation, is shown below.
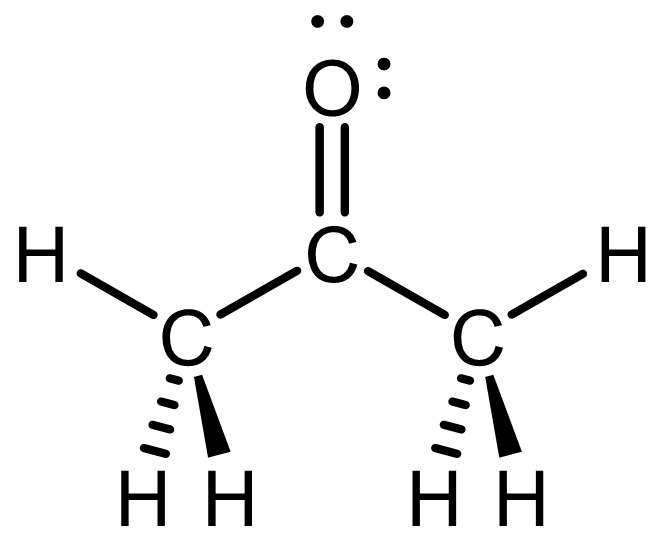
This is one possible perspective; you can draw the acetone molecule in other perspectives (for example, you can choose the perspective where the C=O is pointing down). Click here to see a rotatable 3D structure of acetone; rotate the structure until it matches your drawing.
For simplicity, always draw as many bonds as possible in the plane of the paper/screen. That is, maximize the number of bonds drawn with lines (instead of wedges or dashes).
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

