D4.4 Periodic Variation in Electron Affinities
Electron affinity (EA) is defined as the change in energy when an electron is added to an atom to form an anion. The first EA corresponds to adding one electron to an atom:
Exercise: Ionization Energy vs Electron Affinity
Many elements have negative EA1, which means that the energy of the 1− anion is lower than the energy of the neutral atom plus the free electron; that is, the anion is more stable.
For other elements, EA1 is positive, meaning that the anion is less stable compared to the neutral atom plus a free electron. For these elements, an input of energy is required to form the anion, and, in the gas phase, the anion readily dissociates to yield the neutral atom and a free electron because the latter are lower in energy.
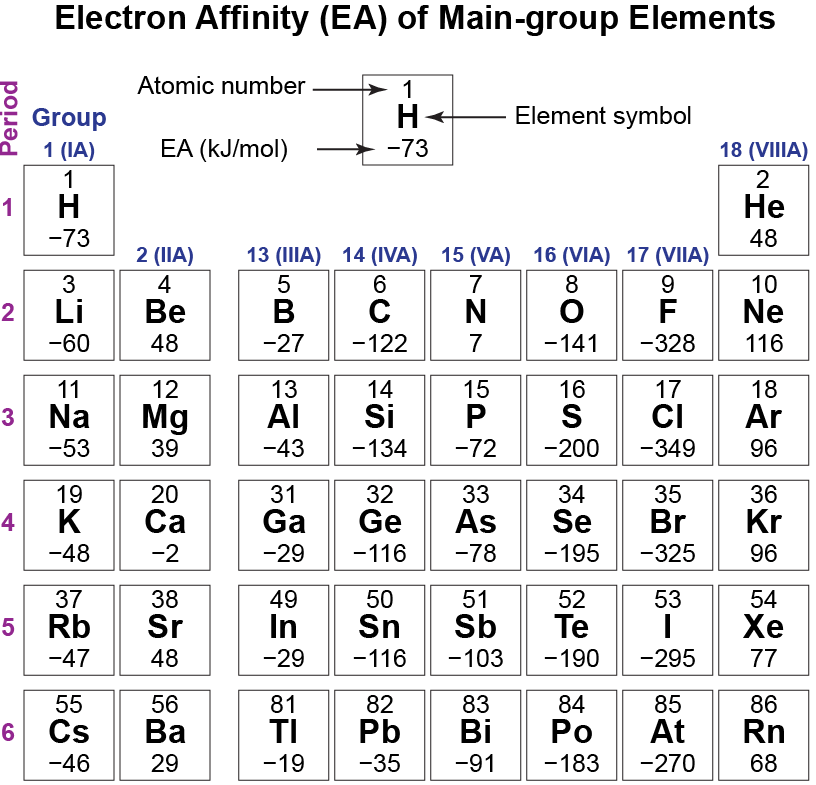
Analyze the data in the table below, which shows electron affinity values (in kJ/mol) for main-group elements. Apply ideas about electron configurations and energies of subshells to explain the trends, and exceptions to trends, in electron affinities. (In earlier Activities, you applied these ideas to trends in ionization energies.) Describe the trends and write explanations for them in your notebook.
Write in your notebook, then left-click here for an explanation.
The main trend going from left to right across a period is that EAs become more negative (atoms attract an external electron more strongly) and the halogens have the most negative electron affinities, but there are many exceptions. Going from top to bottom of the periodic table the trend is different in different periodic groups but, in all but one case, atoms of elements in the second period (Li to Ne) have EAs that are less negative (or more positive) than atoms in the third period (Na to Ar).
There are three exceptions to the trend of increasingly negative EA across the periodic table: group 2 (IIA), group 15 (VA), and group 18 (VIIIA). Atoms in group 2 (IIA) have a filled s subshell, so the next electron added goes into a higher-energy p subshell, making the added electron less stable and the EA less negative. Atoms in group 15 (VA) have a half-filled p subshell and the next electron must be paired with an existing p electron. Increased electron-electron repulsions make that electron less stable and the EA less negative. Atoms of the noble gases, group 18 (VIIIA), have a complete octet and the additional electron must be added to a higher n level that is farther from the nucleus, making that electron less stable and the EA positive.
An example of the second trend is this: EA(F) = -328 kJ/mol but EA(Cl) = -349 kJ/mol. (Cl has the most negative EA of any element.) When a fluorine atom gains an electron to form F–, the electron is in the n = 2 shell; when a chlorine atom gains an electron to form Cl–, the electron is in the n = 3 shell. The n = 3 shell is significantly larger than the n = 2 shell, so there is less repulsion among electrons in the larger shell. The electron added to the smaller n = 2 shell is less stable than the electron added to the n = 3 shell and the chlorine atom accepts an additional electron more readily. The exception to this trend is Li (EA = -60 kJ/mol) and Na (EA = -53 kJ/mol).
There are so many exceptions that it is much more difficult to use the periodic table to predict relative electron affinities than to predict relative radii or ionization energies.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

