D35.2 Polyprotic Acids
We can classify acids by the number of protons per molecule that they can donate in an acid-base reaction. Acids that contain one ionizable hydrogen atom per molecule are called monoprotic acids. Examples include HCl, HNO3, CH3COOH, and HCN.
Even though it contains a total of four hydrogen atoms, acetic acid is monoprotic because only the hydrogen atom in the carboxyl group (-COOH) reacts with bases:
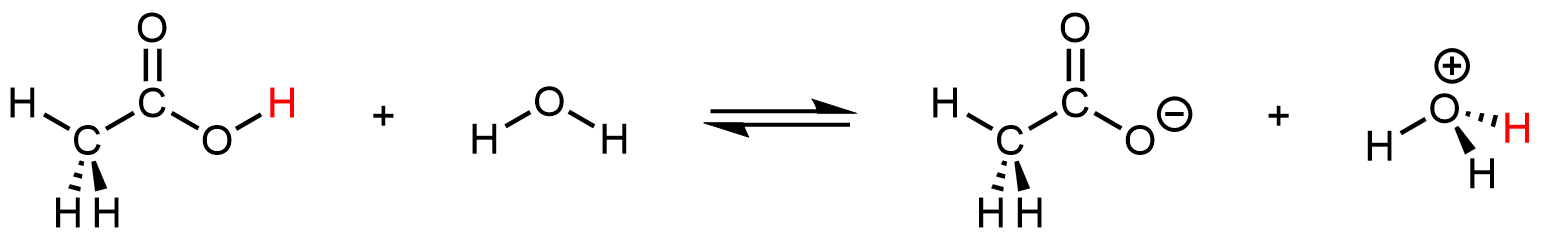
The three hydrogen atoms in the methyl group are not reactive (the C–H bonds are similar those in alkanes, which are generally unreactive).
In the same vein, monoprotic bases are bases that can accept a single proton.
Diprotic acids contain two ionizable hydrogen atoms per molecule. The dissociation of the first H+ always takes place to a greater extent than the dissociation of the second H+, and this is generally true for all polyprotic acids. For example, sulfuric acid ionizes in two steps:
HSO4–(aq) + H2O(ℓ) ⇌ SO42-(aq) + H3O+(aq) Ka,2 = 1.1 × 10-2
This stepwise ionization occurs for all polyprotic acids.
Ka,2 is smaller than Ka,1 because the second acid-base reaction involves an anion (negatively charged molecule) losing a H+ and becoming even more negatively charged. For instance, SO42- contains two excess electrons, and this build up of excess charge destabilizes the molecule. A less stable conjugate base yields a weaker acid, hence HSO4– is a weaker acid than H2SO4.
A solution of a weak diprotic acid contains a mixture of acids. For example, when carbonic acid loses one H+, it yields hydronium ions and bicarbonate ions in small quantities:
The bicarbonate ions can also lose an H+ to form hydronium ions and carbonate ions in even smaller quantities:
Ka, H2CO3 is larger than Ka, HCO3– by about four orders of magnitude (10,000 times larger), so H2CO3 is the dominant producer of H3O+ in the solution. This means that the concentrations of H3O+ and HCO3– are practically equal in a pure aqueous solution of H2CO3.
If the Ka,1 of a weak diprotic acid is at least 20 times larger than its Ka,2, it is appropriate to treat the first ionization separately and calculate concentrations resulting from it before calculating concentrations of species resulting from subsequent ionization. This can simplify the math considerably because we can determine the concentration of H3O+ and the conjugate base from the first ionization, then determine the concentration of the conjugate base of the second ionization in a solution with concentrations determined by the first ionization.
Activity: Ionization of a Diprotic Acid
A triprotic acid is an acid that has three protons that undergo stepwise ionization: Phosphoric acid is an example:
H2PO4–(aq) + H2O(ℓ) ⇌ HPO42-(aq) + H3O+(aq) Ka,2 = 6.3 × 10-8 M
HPO42-(aq) + H2O(ℓ) ⇌ PO43-(aq) + H3O+(aq) Ka,3 = 4.6 × 10-13 M
Again, the differences in the ionization constants of these reactions tell us that the degree of ionization is significantly weaker in each successive step. Here, because the successive ionization constants differ by a factor of 105-106, the calculations of equilibrium concentrations in a solution of H3PO4 can be broken down into parts, similar to the activity above.
Polyprotic bases can accept more than one H+. The carbonate ion is an example of a diprotic base, because it can accept up to two protons. Solutions of alkali metal carbonates (e.g. K2CO3) are quite alkaline, due to the reactions:
HCO3–(aq) + H2O(ℓ) ⇌ H2CO3(aq) + OH–(aq)
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

