D13.4 Stereoisomers: Enantiomers
Another type of stereoisomer is a pair of molecules that are mirror images of each other but cannot be superimposed. Such stereoisomers are called enantiomers (or optical isomers), and are described as being chiral (from the Greek word cheir, χειρ, meaning “hand”). Your left and right hands exhibit chirality—they are non-superimposable mirror images of each other.
Most physical, chemical, and physiological properties of two enantiomeric substances are identical. Differences between enantiomers only become evident when the substances interact with other chiral molecules or chiral environments. (An infamous example of a physiological difference is the drug thalidomide, where one enantiomer causes birth defects but the other does not.)
Most chiral molecules have at least one atom that is bonded to four different groups, a chiral center. Chiral centers are sometimes marked with an asterisk (*) in molecular structures. A carbon atom that is a chiral center is referred to as an asymmetric carbon atom or a chiral carbon atom. For example, the carbon atom in bromochloroiodomethane is a chiral center. There are two enantiomeric isomers: the two mirror-image molecules are not superimposable on top each other.
Activity: Analyzing Enantiomers
Study the two preceding figures carefully. Make certain that you understand what superimpose means and why these two mirror-image molecules cannot be superimposed.
- Based on your observations, devise a method for distinguishing one structure from the other; that is, describe how you can tell that one molecule is different from the other. Write your method in your notebook.
- To convert from (R)-bromochloroiodomethane to (S)-bromochloroiodomethane, is it necessary to break one or more covalent bonds so that atoms could be re-arranged? If so, describe which bonds could be broken and which formed to interchange the structures.
Write in your notebook, then left-click here for an explanation.
There are at least two ways to consider how these two enantiomers differ from each other.
- In Figure B above, both molecules are shown with the C-H bond pointing up. If you look down the C–H bond at the bottom three corners of the tetrahedron and go clockwise starting at I, the left molecule has the order of I, Br, then Cl; the right molecule has the order of I, Cl, then Br.
- Alternatively, you can line up two of the bonds, such as the C-H and C-I bonds as shown after you move the slider to the right in Figure B, and see that the left molecule has the C-Br bond coming up out of the plane, while the right molecule has the C-Cl bond coming up out of the plane. Stop the animation in Figure A and rotate the 3D models to compare and see how the two enantiomers differ.
There are at least two ways to convert one molecule into the other.
- If any two bonds are broken and the bonded atoms interchanged, the result is the other enantiomer. For example, breaking the C-Cl bond and the C-Br bond would allow interchanging Cl and Br atoms. In number 2 above, one enantiomer has Cl sticking out of the plane while the other had Br sticking out. Interchanging the atoms interchanges the isomers, Completely breaking two sigma bonds requires a lot of energy and therefore the enantiomers do not interchange at room temperature.
- A second way to interchange the two atoms is to flip one side of the molecule relative to the other. This would not obviously break bonds, but half way through the rotation the molecule would be square planar rather than tetrahedral. The most stable arrangement for sp3 hybrid orbitals is to point to the corners of a tetrahedron, so this geometry change would raise the energy of the molecule significantly. The energy increase is big enough that enantiomers do not interchange at room temperature.
The “(R)-” and “(S)-” prefixes in their names of the enantiomers indicate that these two substances differ from one another. (In this course we will not deal with how to figure out which prefix goes with which structure.)
The difference between the two enantiomers may not appear to be significant, but you would have to at least partially break and re-form two σ bonds in order to go from one enantiomer to the other. Hence, enantiomeric structures represent different substances that can be separated from one another and would not readily convert from one to the other. Separating enantiomers is often difficult and requires a chiral environment: a different chiral substance that interacts differently with the left-hand molecule compared to the right-hand molecule. A macroscopic analogy is this: if all the gloves you own were mixed up, you could separate left-hand gloves from right-hand gloves by how they interact with your right hand; all gloves that fit are right-handed.
All molecules have a mirror image, but only chiral molecules are not superimposable on top of their mirror images. Contrast CHBrClI with CHCl2I (dichloroiodomethane), which is shown in the next figure. CHCl2I and its mirror image are superimposable. Thus CHCl2I is not a chiral molecule (it is said to be achiral). Two groups (the Cl atoms) bonded to the carbon atom in CHCl2I are the same, so there is no chiral center.
Usually, the easiest way to spot a chiral center is to look for four different groups bonded to an atom. The “groups” are considered in their entirety, not just the atom that is directly bonded to the chiral center. For example, a methyl group is a different group from an ethyl group, which is different from a propyl group and so forth. Hence, alkanes can be chiral, for example:
Do not automatically assume that you are looking at a chiral center just because you see dashed and solid wedges in a structure. For example, lactic acid has an asymmetric (chiral) carbon atom (denoted by *) and is a chiral molecule, while isobutyric acid is not a chiral molecule.
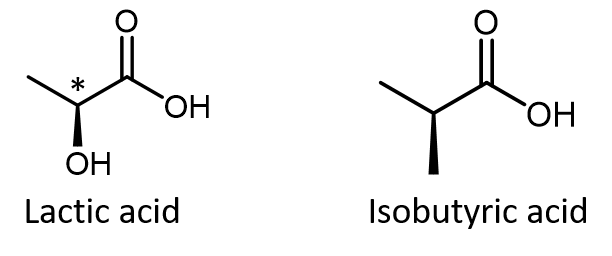
Make sure you can recognize from the wedge-dash Lewis structure that the chiral carbon atom in lactic acid has four different groups bonded to it but the corresponding carbon atom in isobutyric acid does not.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

