D28.2 Unimolecular Elementary Reactions
A unimolecular elementary reaction involves the rearrangement of a single reactant molecule to produce one or more product molecules. The cis–trans isomerization of 1,2-difluoroethene is an example of a unimolecular elementary reaction.
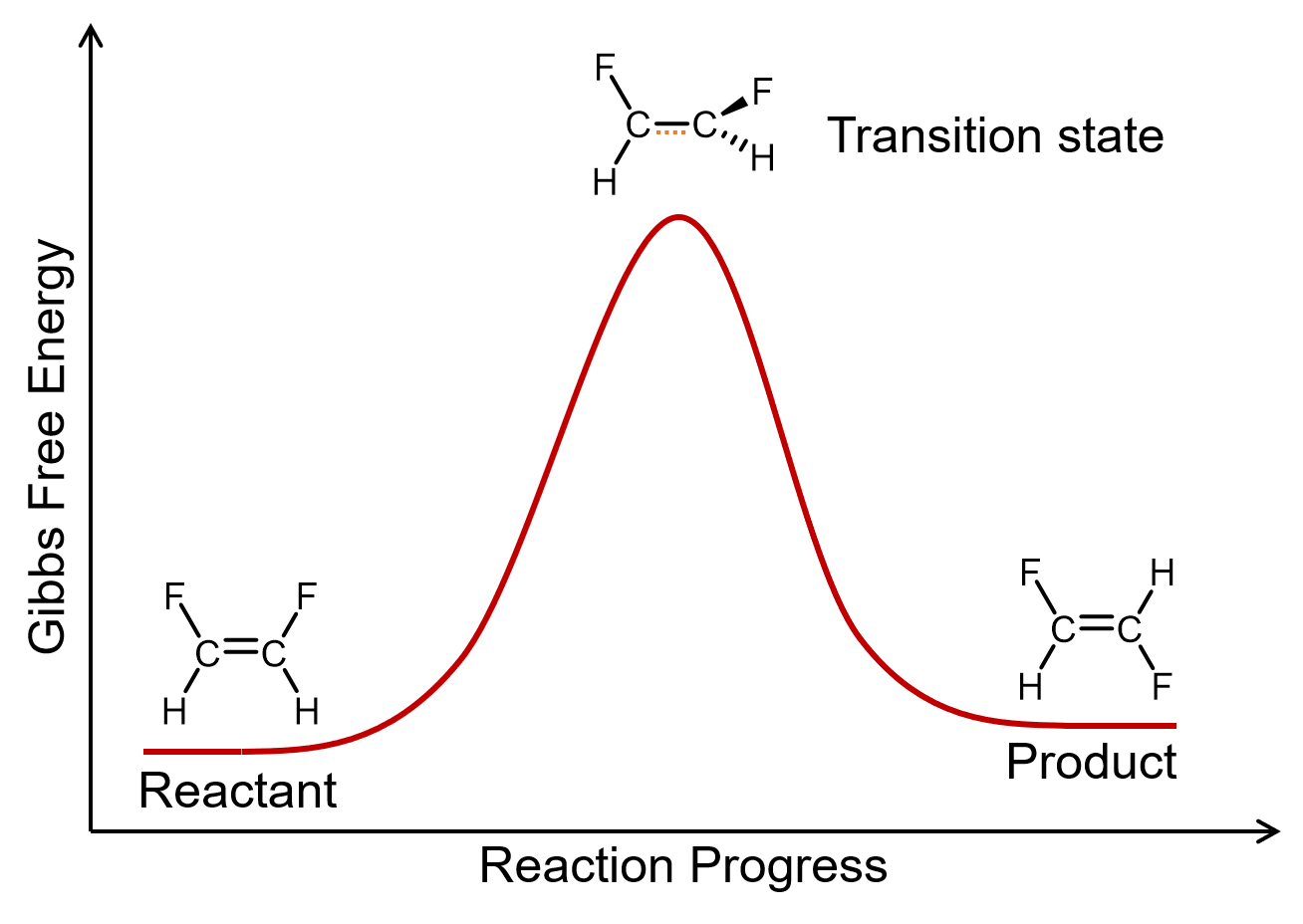
A general equation for a unimolecular elementary reaction is:
Suppose that a 1.0-L solution contains 0.0010 mol of reactant A. The concentration of A is 0.0010 mol/1.0 L = 0.0010 M. At a given temperature, a tiny fraction of the A molecules has enough energy to overcome the activation-energy barrier and react. If we add another 0.0010 mol of A to the flask, the number of A molecules doubles and the concentration of A doubles to 0.0020 M. If the temperature remains the same, the fraction of molecules that has enough energy to react remains the same, but now there are twice as many molecules so the number of molecules reacting (and the rate of reaction) doubles; that is, the rate of reaction is directly proportional to the concentration of A:
Exercise: Unimolecular Reactions
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

