D17.4 Solutions and Solubility
A solution is a homogeneous mixture of two or more substances. Often, one component of a solution is present at a significantly greater concentration, in which case it is called the solvent. The other components of the solution, present in relatively lesser concentrations, are called solutes.
When solute and solvent are in the gas phase, the substances are always completely miscible, which means they have infinite mutual solubility. In the gas phase, molecules are far apart relative to their sizes, so IMFs (intermolecular forces) are negligible. A mixture of solute molecules with solvent molecules is more probable than having the molecules remain separated, so the molecules mix. This higher probability of a mixture is related to entropy, a property we will discuss later in the course.
For liquid-phase solutions the molecules are in close contact and IMFs affect solubility. In separate, pure samples of solute and solvent, solute molecules all contact other solute molecules and solvent molecules all contact other solvent molecules, so there are only solvent-solvent and solute-solute IMFs. When a few solute molecules are dispersed among many solvent molecules, almost all solute molecules are surrounded by solvent molecules and solute-solute IMFs are replaced by solute-solvent IMFs.
An example of the effect of IMFs is solubility of a hydrocarbon, such as hexane, in water. In hexane the only IMFs are London forces whereas in water the most important IMFs are hydrogen bonds, and there are also dipole-dipole and London forces. Water molecules are not strongly attracted to hexane molecules: London forces are small because a water molecule has only 10 electrons; hexane molecules do not participate in hydrogen bonding. Thus, the water-water IMFs and hexane-hexane IMFs are much stronger than hexane-water IMFs. Dissolving hexane in water would require significant input of energy (dissolving hexane in water is an endothermic process with positive enthalpy change).
Only a small quantity of hexane can dissolve in water before this energy increase counteracts the tendency of the molecules to mix. The solubility of hexane in water is only 0.01 g per hundred grams of water. Only a small quantity of water can dissolve in hexane for the same reason and the solubility of water in hexane is (coincidentally) only 0.01 g/100 g. Mixing more than 0.01 g of either substance with 100 g of the other results in two very dilute solutions: one is a dilute solution of hexane in water; the other is a dilute solution of water in hexane. Hexane and water are immiscible, which means that they do not mix completely but rather form two separate liquid layers. Hexane is said to be hydrophobic (“water-fearing”), because the intermolecular attractions of its molecules with water molecules are weak.
Exercise: Explaining Water Solubility of Alcohols
The -OH functional group of an alcohol molecule is hydrophilic (“water-loving”), capable of strong intermolecular attractions to water molecules. This is similarly true for other polar and hydrogen-bonding functional groups. For example, acetic acid is miscible with water, while octanoic acid is immiscible with water.
Aqueous Solutions of Ionic Compounds
The situation is slightly different when an ionic compound (usually a solid) is added to water. For example, when solid potassium chloride, KCl, is added to water, the compound dissolves and dissociates to yield K+(aq) and Cl–(aq) uniformly distributed throughout the mixture:
For all ionic compounds a solubility limit is eventually reached: if enough solid is added to water, the solution becomes saturated. Adding more of the ionic compound does not increase the solution concentration and some solid remains undissolved.
ln an ionic solid the forces holding particles together are attractions among charged ions. These attractions give rise to the lattice energy, which directly affects the solubility. Dissolving an ionic compound requires breaking apart the ionic lattice (an endothermic process, ΔH > 0). This energy increase can be partially or completely compensated by attractions between ions and the dipoles of polar molecules. For example, the negative ends of water-molecule dipoles are strongly attracted to positive ions. This reduces the energy (and enthalpy) of the solution by forming new water-ion interactions (an exothermic process).
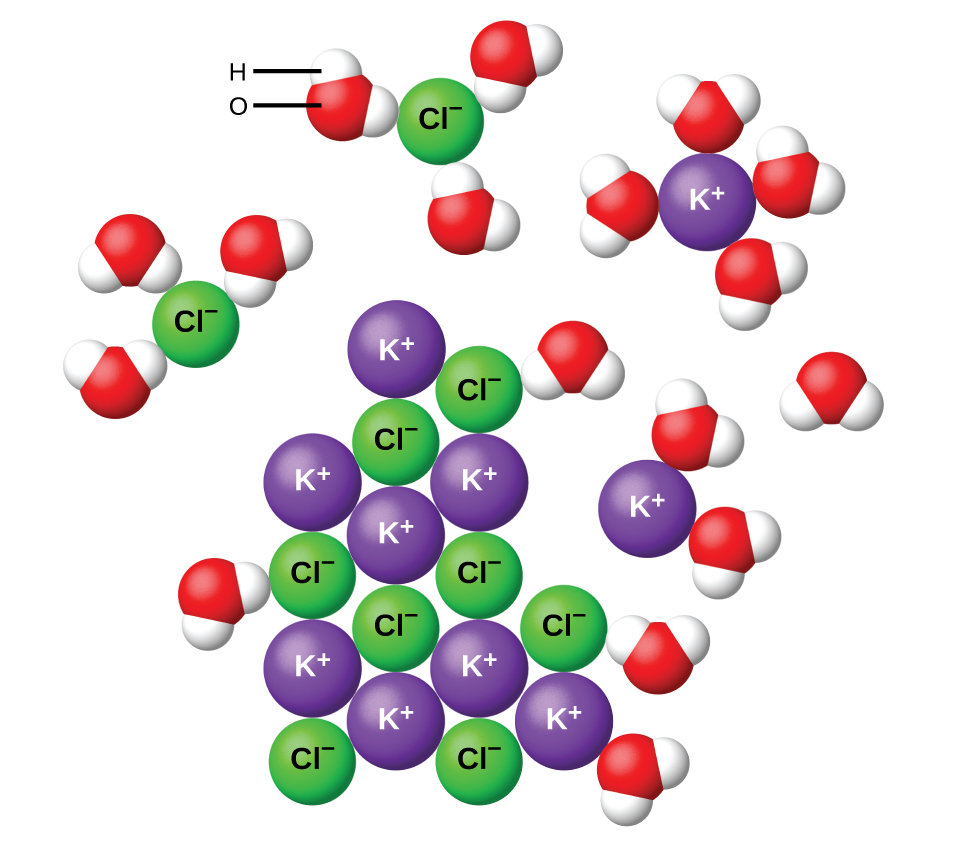
The lattice energy and the water-ion interaction strength (solvation energy) are often comparable in magnitude. Therefore, dissolving an ionic compound in water is usually slightly endothermic or slightly exothermic overall. Ionic compounds that dissolve exothermically are generally quite soluble. On the other hand, ionic compounds that dissolve endothermically are not always insoluble (see video below). This is a result of the entropy factor that was mentioned at the beginning of this section.
Exercise: Solubility of Ionic Compounds in Water
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

