D8.1 LDFs and Molecular Behavior
When a molecular substance exists as a gas, its particles are far apart, with few interactions holding them together. As molecules become larger, have more electrons, and become more polarizable, LDFs between them become stronger. That allows the substance to condense into a liquid (or even solidify) at a higher temperature.
- Methane (CH4) boils at -162 °C
- Hexane (C6H14) boils at 69 °C
- Octadecane (C18H38) is a waxy solid at room temperature and melts at 28-30 °C.
The reason? Octadecane has more electrons and a longer molecular surface area, so its LDFs are stronger, requiring more thermal energy to overcome. LDFs grow stronger when:
- There are more electrons in the system (larger atoms or molecules)
- The electron density is more polarizable (easier to distort)
- Molecules have more surface area in contact (long, floppy shapes help).
These differences in physical state and melting/boiling point do not arise from chemical bonds within the molecules, but rather from interparticle interactions (interactions between particles)—LDFs that we know how to reason using Coulombic attraction and electron distribution.
Activity
Examine the boiling points of hexane and octadecane. What features of their molecular structures contribute to the difference? How would you expect nonane (C9H20) to behave?
Draw and write in your notebook, then left-click here for an explanation.
An octadecane molecule has more electrons than a hexane molecule. Molecules of octadecane also have greater surface area in contact when they are close to each other.
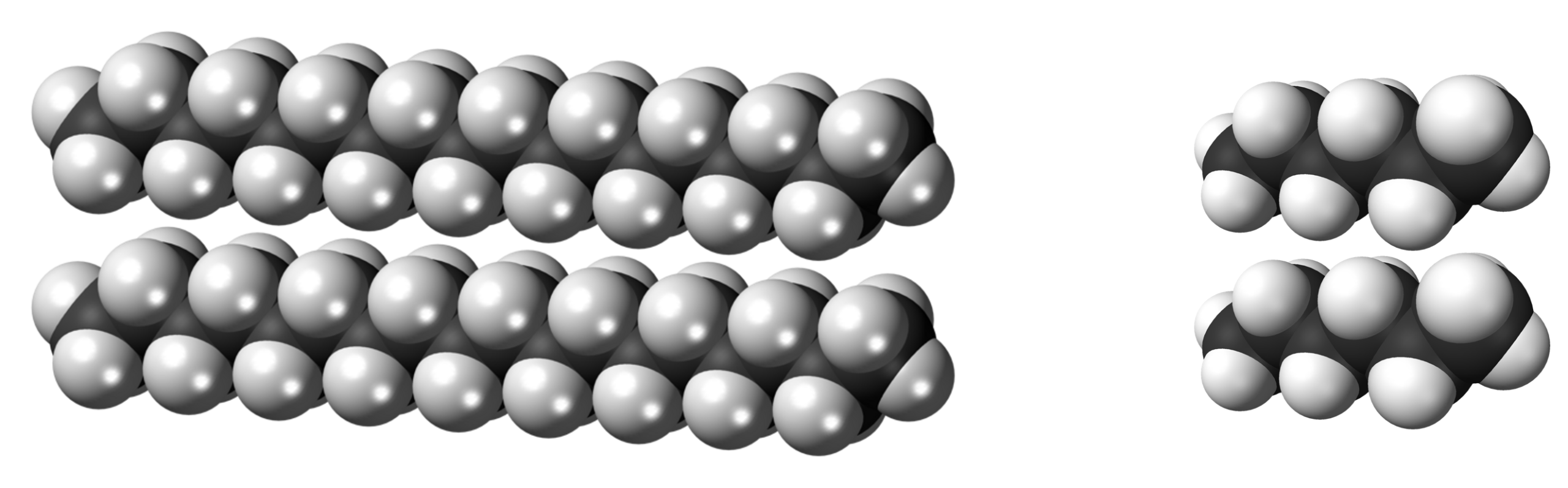
Therefore, octadecane has stronger LDFs, leading to its boiling point (317 °C ) being higher than that of hexane.
Nonane has more electrons than hexane but less than octadecane. It's a longer hydrocarbon molecule than hexane, but shorter than octadecane. Therefore, its LDFs are likely stronger than those in hexane but weaker than those in octadecane, and its boiling point temperature would be in between those of hexane and octadecane. (Nonane's boiling point is 151 °C).
Remember, LDFs arise from interactions between partial charges. Even if those charges are brief and constantly shifting, they still obey Coulomb’s Law:
[latex]E = k \cdot \dfrac{q_1 q_2}{r}[/latex]
This means the closer two partial charges are and the larger they are, the stronger the attractive force. Larger atoms and molecules:
- have more electrons → greater fluctuations.
- are more polarizable → easier to distort.
- have greater contact area
This is why, at room temperature, iodine (I2), which has 106 electrons, is a solid, while fluorine (F2), with only 18 electrons, is a gas.
| Alkane | Molecular Formula | Number of Electrons | Melting Point (°C) | Boiling Point (°C) | Phase at Room Temperature |
|---|---|---|---|---|---|
| methane | CH4 | 10 | –182.5 | –161.5 | gas |
| ethane | C2H6 | 18 | –183.3 | –88.6 | gas |
| propane | C3H8 | 26 | –187.7 | –42.1 | gas |
| butane | C4H10 | 34 | –138.3 | –0.5 | gas |
| pentane | C5H12 | 42 | –129.7 | 36.1 | liquid |
| hexane | C6H14 | 50 | –95.3 | 68.7 | liquid |
| heptane | C7H16 | 58 | –90.6 | 98.4 | liquid |
| octane | C8H18 | 66 | –56.8 | 125.7 | liquid |
| nonane | C9H20 | 74 | –53.6 | 150.8 | liquid |
| decane | C10H22 | 82 | –29.7 | 174.0 | liquid |
| tetradecane | C14H30 | 114 | 5.9 | 253.5 | solid |
| octadecane | C18H38 | 146 | 28.2 | 316.1 | solid |
| Table: Melting and boiling points of alkanes. | |||||
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

