D8.2 Molecular Substances vs. Extended Solids
Some substances, like hydrocarbons or halogens, are made of discrete molecules. Others, like diamond or graphite, consist of extended bonding frameworks where each atom is bonded to others in a continuous pattern.
The type and dimensionality of bonding dramatically changes a substance’s macroscopic properties:
| Substance | Type of Structure | Key Bonding Type | Boiling/Melting Behavior |
| Methane (CH4) | Molecular gas at room temp | Covalent within CH4 + LDFs between | Boils at –161 °C |
| Iodine (I2) | Molecular solid at room temp | Covalent within I2 + LDFs between | Boils at 184.3 °C |
| Graphite | Extended 2D | Covalent in planes + LDFs between | Sublimes ~3650 °C (no melting) |
| Diamond | Extended 3D | Covalent throughout | Sublimes ~3600-3900 °C |
| Fullerene (C60) | Molecular solid at room temp | Covalent in C60 + LDFs between | Sublimes ~527 °C |
Graphite and diamond are both made entirely of carbon, yet their bonding dimensionality:
- Diamond is 3D—bonds go in all directions.
- Graphite is 2D—strong bonds in flat sheets, but LDFs between layers.
gives rise to completely different properties:
- Diamond is rigid, transparent, and an electrical insulator.
- Graphite is soft, opaque, and conducts electricity along the planes.
Activity: graphite vs. diamond 1
Why can you “write” with graphite but not diamond? In other words, why can graphite leave marks on paper, but diamond cannot? Use bonding dimensionality and intermolecular vs. covalent bonding to explain.
Write in your notebook, then left-click here for an explanation.
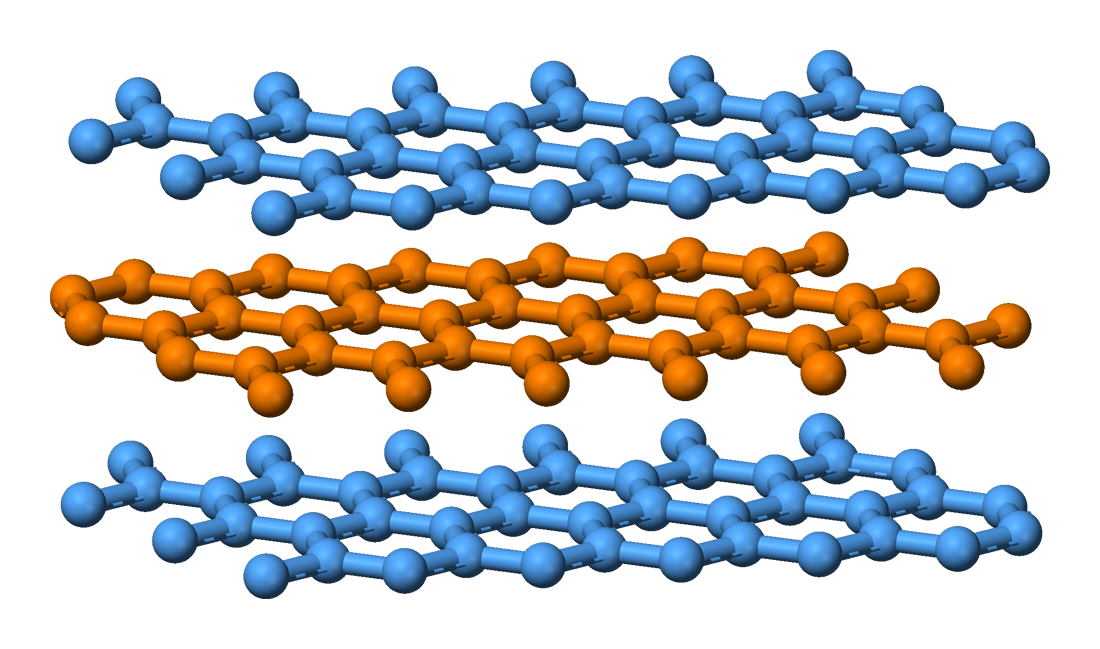 LDFs hold flat sheets of graphite (the individual sheets are called graphene) together. These LDFs can be overcome relatively easily, allowing the layers to glide past one another and be separated with application of force from your hand. These layers are left behind on the paper (held in place by LDFs).
LDFs hold flat sheets of graphite (the individual sheets are called graphene) together. These LDFs can be overcome relatively easily, allowing the layers to glide past one another and be separated with application of force from your hand. These layers are left behind on the paper (held in place by LDFs).
Diamond has covalent bonds in all directions. In order to separate pieces of diamonds, covalent bonds need to be broken, which requires more energy.
Allotropes of Carbon: A Case Study in Bonding Dimensionality
Carbon’s ability to form different types of bonds enables the existence of a variety of allotropes—different forms of the same element.
| Allotrope | Dimensionality | Bonding | Physical Behavior | Melting/Sublimation Point |
| Diamond | 3D | Covalent network | Transparent, very hard, electrical insulator | Sublimes ~3600-3900 °C |
| Graphite | 2D + LDFs | Covalent in sheets | Opaque, slippery, conducts electricity along planes | Sublimes ~3627 °C |
| Fullerenes (C60) | 0D | Molecular (discrete) | Forms soft molecular solids, electron-rich | Sublimes ~527 °C |
| Carbon Nanotubes | 1D | Covalent extended | Strong, flexible, conduct heat and electricity, often cylindrical | Decomposes ~600–1000 °C |
Why does graphite feel greasy, while diamond is abrasive? Why can you melt pentane, but not melt graphite?The key is interaction scale: molecular substances have comparably weaker LDFs between individual molecules. These are easier to overcome with heating. But in extended structures, each atom is covalently bonded to others. It takes enormous energy to break those bonds, which is in part why diamond and graphite sublime rather than melt. This also explains trends in melting and boiling across carbon-containing substances:
- C4H10 (butane): Boiling point = 0 °C
- C18H38 (octadecane): Melting point = 28 °C
- Graphite/Diamond: Sublimation 3600-3900 °C
| Feature | Molecular Substances | Extended Solids |
| Bonding Type | Covalent within molecules, LDFs between | Covalent throughout the entire structure |
| Typical Examples | Hydrocarbons, O2, I2, fullerenes | Graphite, diamond, carbon nanotubes |
| Melting/Boiling Points | Low to moderate | Extremely high (often sublime) |
| Electrical Conductivity | Usually insulators | Varies (graphite: conductor; diamond: insulator) |
| Dimensionality of Interaction | 0D (molecules) | 2D (graphite), 3D (diamond), 1D (nanotubes) |
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

