D7.4 Charged Hydrocarbon Ions: Structure vs. Stability
So far, every molecule we’ve seen has followed the same pattern: each carbon atom is surrounded by four electron pairs (two electrons per bond), and each hydrogen is connected by just one. That pattern is not arbitrary—we observe it because it’s based on stable molecules, i.e., those that are not reactive under normal conditions.
In many stable molecules, we often observe atoms (especially in the second period, like carbon) surrounded by eight electrons, thus filling their valence shell. (This is often called the “octet rule”.) For hydrogen, only two electrons are needed to fill its valence shell.
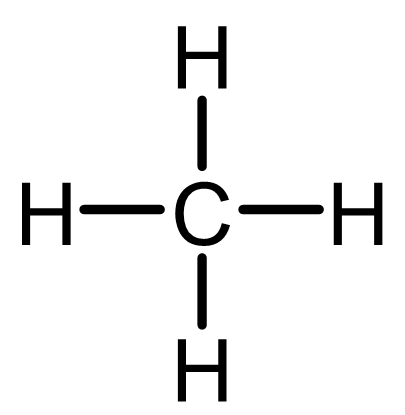 In methane (CH4), this is easy to see: carbon forms four single bonds to hydrogen. Each bond shares a pair of electrons, giving carbon access to a full octet.
In methane (CH4), this is easy to see: carbon forms four single bonds to hydrogen. Each bond shares a pair of electrons, giving carbon access to a full octet.
But what happens when a molecule gains or loses electrons? Let’s consider two modified forms of methane:
 |
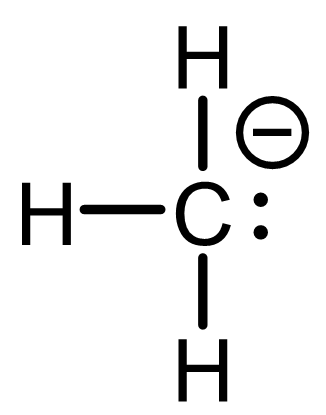 |
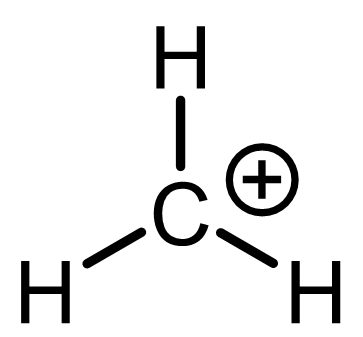
| Remove a hydrogen and both bonding electrons (i.e., an H¯), and you get CH3+, a carbocation. Now carbon is only surrounded by three bonds—just six electrons, not eight. | Remove a proton (H+) and you get CH3¯, a carbanion. In this case, the electrons that formed the C-H bond become a lone electron pair on carbon, giving it eight electrons—but an excess of electron density (a negative charge). |
To consider these two Lewis structures further, we can utilize one more tool—formal charge. Formal charge helps us evaluate the electron distribution in a structure based on how many electrons an atom “owns” in the structure, compared to how many it typically brings to the table.
Formal charge assumes that electrons are shared evenly between the two atoms in the bond; for the C-H and C-C bonds in hydrocarbons, this is not a terrible assumption (a point we will return to in Unit 2). So, let’s look more closely, assuming each atom in the bond “owns” one electron in that bond:
- In CH4, carbon makes four bonds and shares one electron in each. That’s four electrons “owned” by the carbon atom, which matches the four valence electrons a carbon atom has, given its position in the periodic table. So the formal charge on carbon is 0.
- In CH3+, carbon makes only three bonds—meaning it “owns” just three electrons. That’s one fewer than expected. So the formal charge on carbon is +1.
- In CH3¯, carbon makes three bonds and has a lone electron pair, for a total of five “owned” electrons. That’s one more than expected. So the formal charge on carbon is -1.
Formal charge helps us quantify how electrically “unbalanced” an atom is in a Lewis structure, even when the structure appears complete. But for the actual molecule, we also care about its stability, and that is more complicated than just following the octet rule or having low formal charges. The Lewis structure of a molecule may appear “reasonable” on paper—octet satisfied, formal charges minimized—but the molecule may still be high in energy.
Left-click here for further information
Sometimes, even a relatively low energy structure can be considered unstable if it readily transforms into something even lower in energy. In other words, stability and energy are connected, but stability requires a full accounting of all energetic considerations, which is seldom easy.
As a general rule of thumb: structures that follow typical bonding patterns, minimize formal charge, and satisfy the octet rule are more likely to be comparably lower in energy, and therefore more likely to be stable. But it’s just that: a rule of thumb. Chemistry is full of exceptions, especially when other structures are available that may be lower in energy.
Consider the following comparisons:
- CH3+ (methyl cation) is quite unstable. The carbon lacks an octet and carries a +1 formal charge. When it reacts with H¯ to form CH4 (i.e., the CH3+ + H¯ ⟶ CH4 reaction), about 1320 kJ/mol of energy would be released
- In other words, CH4 (methane) is 1320 kJ/mol lower in energy than CH3+ + H¯.
- CH3¯ (methyl anion) has a full octet, but it concentrates negative charge on a small atom with no way to stabilize it. When it reacts with H+ to form CH4 (i.e., the CH3¯ + H+ ⟶ CH4 reaction), an even greater 1740 kJ/mol of energy would be released
- In other words, CH4 (methane) is 1740 kJ/mol lower in energy than CH3¯ + H+.
So does this mean CH3+ is more stable than CH3¯? Not quite. These energetic comparisons are not a direct measure of the absolute stabilities of these two molecules because the reactions involve different species (H¯ vs. H+). Energy values like these are relative to all the reaction species in the equation, not absolute comparisons of the charged species themselves.
Instead, here’s the reliable conclusion you can draw: both the methyl cation (CH3+) and the methyl anion (CH3¯) are much higher in energy than their neutral counterpart, methane (CH4). This tells us that charged molecules are often less stable than their neutral counterparts, especially when the charge is concentrated on small atoms without ways to spread it out or stabilize it. That’s why both CH3+ and CH3¯ are highly reactive—they each represent a higher-energy, less stable state that “wants” to return to neutrality.
Stability Patterns in Charged Hydrocarbons: Learning from Reaction Energies
That said, we can explore data to extract patterns of stability by comparing different cations and different anions. For example, consider the reaction of a carbocation with a hydride ion (H¯), which produces a neutral alkane. The amount of energy released in this reaction tells us something about how stable (or unstable) the carbocation is: more energy released means the starting carbocation is higher in energy—less stable.
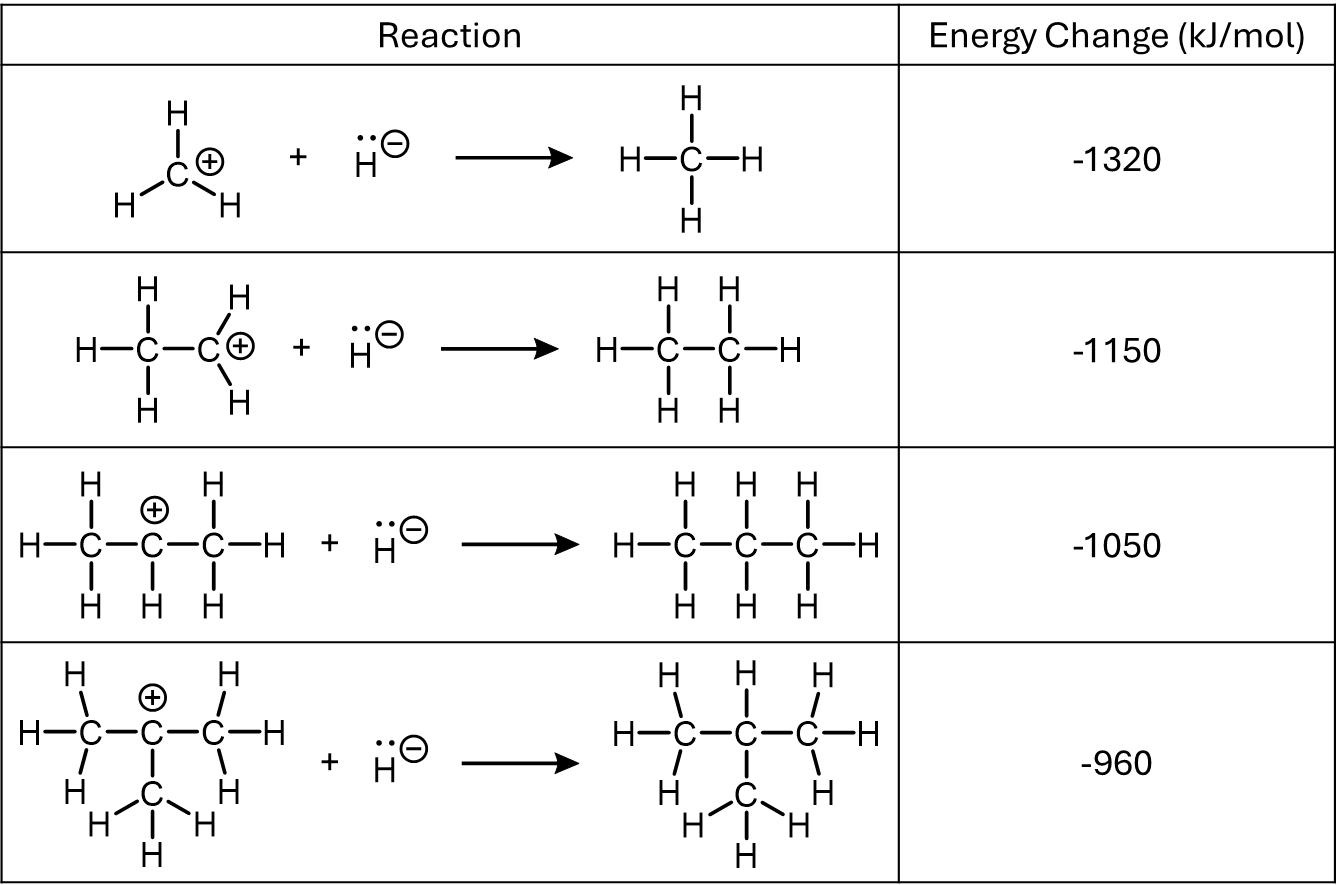
Activity: carbocation stability
Consider the table above:
- What pattern do you notice between the structure of the carbocation and the amount of energy released in the reaction?
- What does that tell you about how carbocation stability changes with substitution?
- Why do you think this trend occurs? In other words, how might the presence of more carbon groups help stabilize the positively charged carbon atom?
Be as specific as you can about where the positive charge is located and how nearby atoms may help stabilize it.
Write in your notebook, then left-click here for an explanation.
Let’s take a closer look at this data. Each row shows a carbocation—a positively charged molecule—reacting with a hydride ion (H⁻) to form a neutral molecule. The number on the right tells us how much energy is released during that reaction. The bigger the number, the less stable the carbocation is—because it had more energy to “give up” when it formed a neutral product.
Now, let’s compare the structures:
- CH3+ (methyl cation) releases 1320 kJ/mol. That’s a lot of energy. It’s just a single carbon with a positive charge and only H atoms around it to help—that’s not a lot of electron density to help offset the positive charge. It’s extremely unstable.
- C2H5+ (primary carbocation) releases 1150 kJ/mol. This time, there’s one additional carbon connected to the positively charged carbon. That’s more electron density nearby—and it makes the cation slightly more stable.
- C3H7+ (secondary carbocation) releases 1050 kJ/mol. Now two carbon atoms surround the positive center. Even more electron density is nearby, and the cation is more stable.
- C(CH3)3+ (tertiary carbocation) releases 960 kJ/mol. Three other carbon atoms surround the central one, and this structure is the most stable of this set.
So, what’s the pattern?
As you increase the number of atoms around the positively charged carbon, the more stable the carbocation becomes. That’s because those surrounding atoms bring more electron density into the region of the molecule where it’s needed most—helping to “soften” or “buffer” the impact of the deficiency in electron density (the positive charge). This does not mean the molecule is more neutral (the overall molecule is still +1 charged), but it does mean the positive charge is less concentrated—and that makes the molecule lower in energy and more stable overall.
This is an important idea in chemistry: charge stability depends not only on whether an atom has a full octet, but also on how well the rest of the molecule helps support that charge.
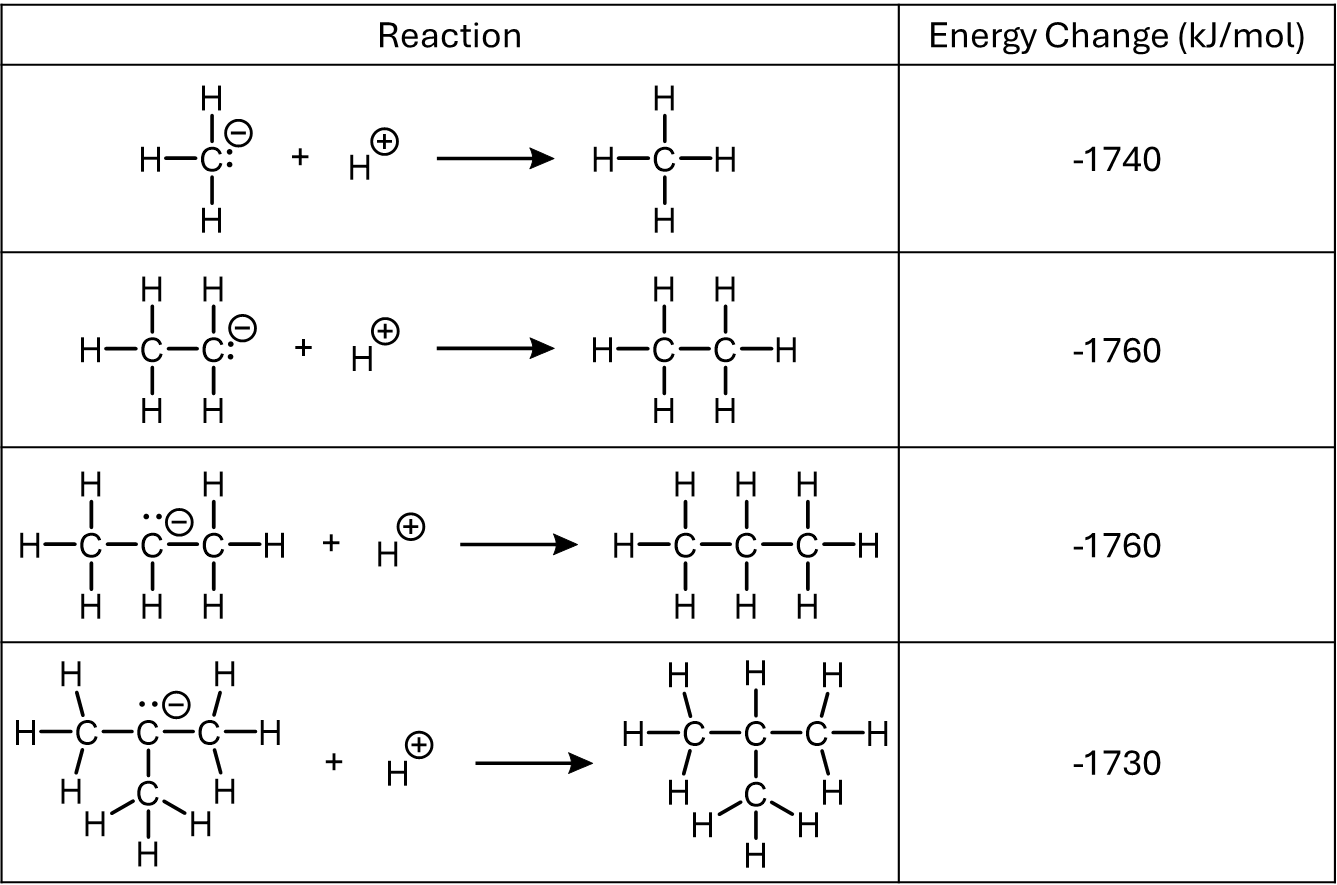
Below is data for a series of carbanions (negatively charged hydrocarbon species), each reacting with a proton (H+) to form a neutral hydrocarbon. As before, the values indicate the amount of energy released during the reaction.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

