Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 8 Pre-Class Podia Activity
Phosphorus can exist in several forms (allotropes). Two common ones are white phosphorus and red phosphorus.
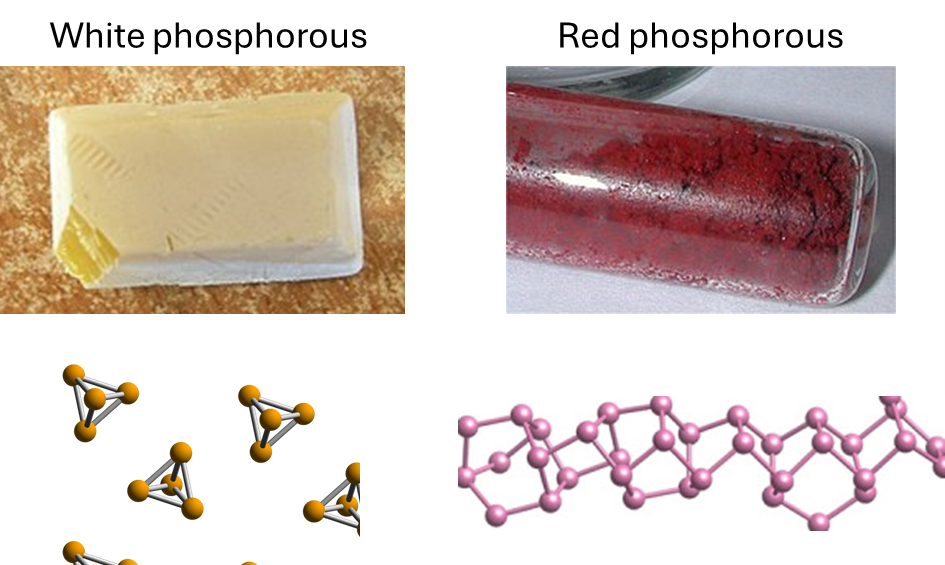
White phosphorus is waxy, glows faintly in the dark, and is dangerously flammable; it bursts into flame when exposed to air. Red phosphorus, in contrast, is stable in air and is used safely on the striking surface of matchboxes.
- Use the structures of white phosphorus and red phosphorus shown above to explain:
- Why white phosphorus is much more reactive than red phosphorus.
- Why red phosphorus is more stable in air.
Hint: consider whether the bonding is molecular or extended, and what this means for bond strain, bond strength, and dimensionality.
- Reflect:
- Why might it be useful for chemists to describe phosphorus as having different “allotropes” instead of just saying “it’s all phosphorus”?
- How does this example illustrate the importance of bonding structure and dimensionality in determining physical and chemical properties?
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

