D14.1 Bond Polarity
If the two atoms that form a covalent bond are identical, as in H2 or Cl2, then the electrons in the bond must be shared equally between the two atoms. In a pure covalent bond, shared electrons have an equal probability of being near each nucleus.
On the other hand, if the two atoms are different, they may have different attractions for the shared electrons. When the bonding electrons are attracted by one atom more than the other atom the bond is called a polar covalent bond. For example, in HCl, the Cl atom attracts the bonding pair of electrons more than the H atom, and electron density of the H–Cl bond is shifted towards the Cl atom. Quantum mechanics calculations show that the Cl atom, which has 17 protons, has electron density equivalent to 17.28 electrons and therefore a partial negative charge, δ– = −0.28. The hydrogen atom has a partial positive charge, δ+ = +0.28.
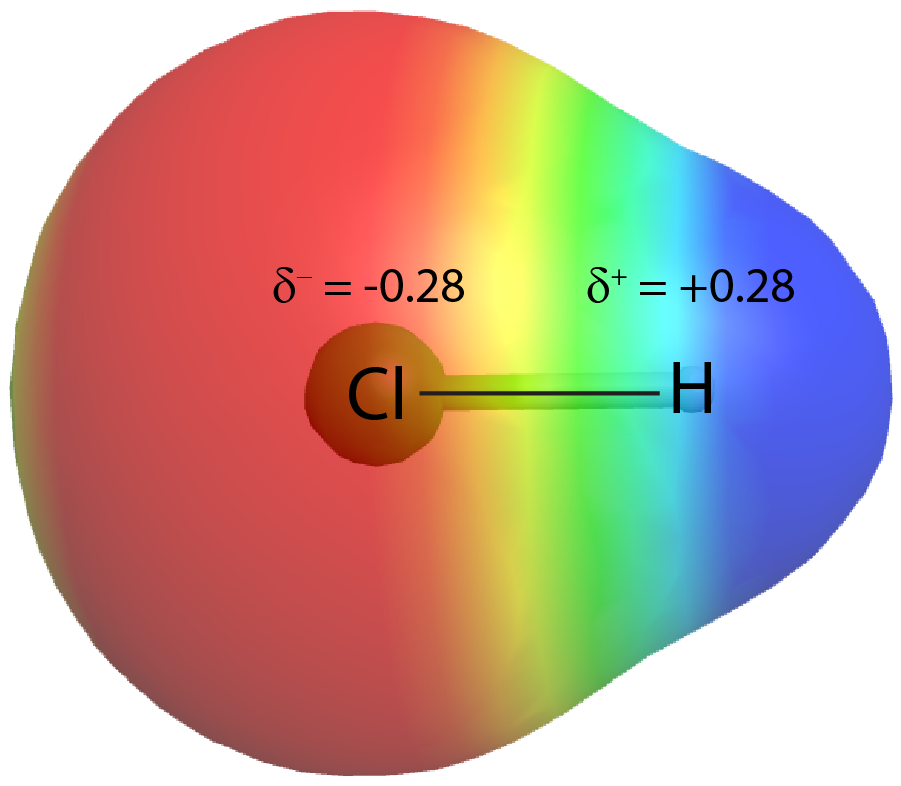
This unequal distribution of electron density on two bonded atoms produces a bond dipole moment, the magnitude of which is represented by µ (Greek letter mu):
where Q is the magnitude of the partial charges (for HCl this is 0.28 times the charge of an electron) and r is the distance between the charges (the bond length). Bond dipole moments are measured in units of debyes (D); 1 D = 3.336 × 10-30 Coulomb·meter.
The bond dipole moment ([latex]\overrightarrow \mu[/latex]) has both direction and magnitude and can be represented as a vector (see Figure: Bond Dipole Moment). A dipole vector is drawn as an arrow, with the arrowhead pointing to the partially negative end, and a small + sign on the partially positive end. The length of the arrow is proportional to the magnitude of the dipole moment.
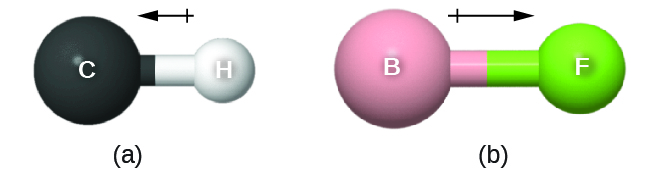
Why is the direction of the arrow different from what I learned in physics?
In physics, by convention, a dipole vector points toward the positive end of the dipole.
In chemistry, electrons (with negative charge) are more important because the position of electron density relative to atomic nuclei defines chemical bonds. Thus, in chemistry, dipoles emphasize the direction of greater electron density and the dipole arrow points toward the negative end of the dipole.
The polarity of a covalent bond can be estimated by the difference between the electronegativities of the bonded atoms. In a bond, the more electronegative atom is the one with the δ− charge. The greater the difference in electronegativity between two bonded atoms, the larger the shift of electron density in the bond towards the more electronegative atom. Greater electronegativity difference, Δ(EN), gives larger partial charges on the atoms.
Electronegativity and Bond Type
The difference in electronegativity, Δ(EN), of two bonded atoms provides a rough estimate of polarity of the bond, and thus of the bond type. When Δ(EN) is very small (or zero), the bond is covalent and nonpolar. When Δ(EN) is large, the bond is polar covalent or ionic. (In a pair of ions, such as Na+Cl−, there is nearly complete transfer of valence electrons from one atom to another to produce a positive ion and a negative ion. The Na+ and Cl− ions form a dipole with δ+ approximately equal to +1 and δ− approximately −1.)
Δ(EN) spans a continuous scale and serves as a general guide; there is no definitive cutoff that defines a bond type. For example, HF has Δ(EN) = 1.8 and is considered a polar covalent molecule. On the other hand, NaI has a Δ(EN) of 1.7 but is considered an ionic compound. When estimating the covalent or ionic character of a bond, you should also take into account the types of atoms involved and their relative positions in the periodic table. Bonds between two nonmetals are usually described as covalent; bonding between a metal and a nonmetal is often ionic.
Some compounds contain both covalent and ionic bonds. For example, potassium nitrate, KNO3, contains the K+ cation and the polyatomic NO3− anion, which has covalent bonds between N and O.
Exercise: Polarity and Electronegativity Difference
Exercise: Bond Polarity and Electronegativity
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

