D36.2 Amino Acids
Structure
An amino acid molecule contains both an amine functional group, which is a weak base, and a carboxylic acid functional group, which is a weak acid. This combination gives amino acids some interesting acid-base properties.
Organic carboxylic acids generally have Ka = ~10-4. Organic amines generally have Kb = ~10-4. The presence of two conjugate acid-base pairs in a single molecule means there are multiple possible structures, ranging from fully protonated:
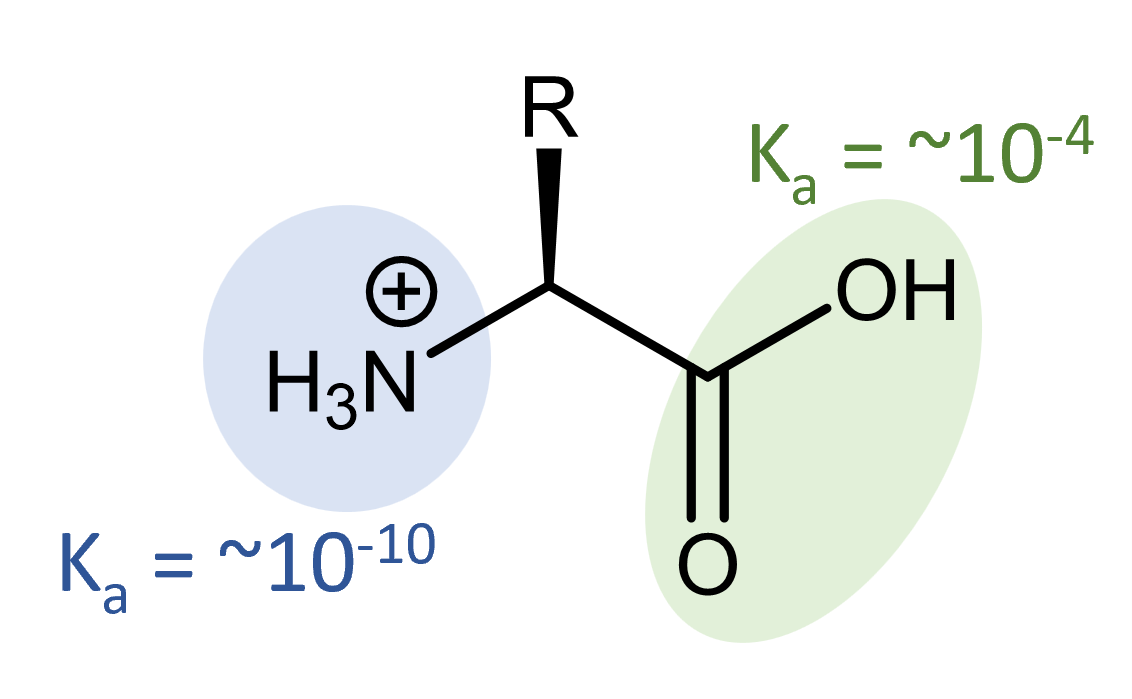
to fully deprotonated.
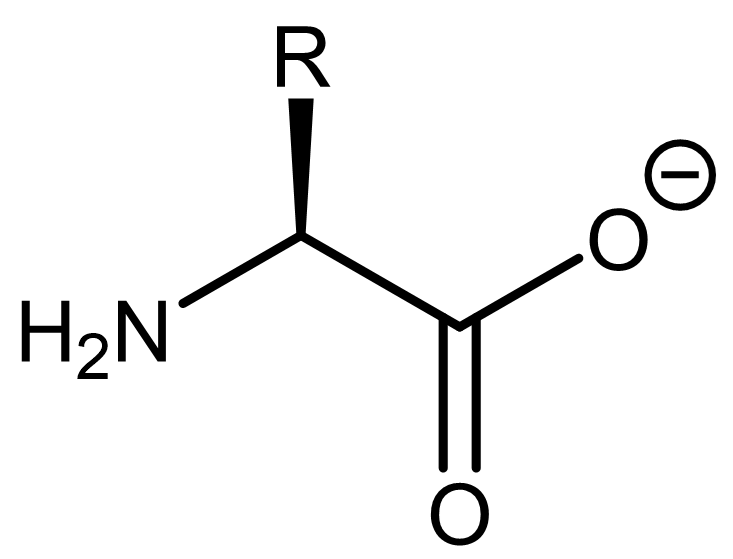
In other words, a fully protonated amino acid is a diprotic acid, and the dominant form the amino acid adopts would depend on the solution’s pH.
For example, the fully protonated alanine has an acidity of Ka,-COOH = 4.6 × 10-3 and Ka, -NH3+ = 1.3 × 10-10.
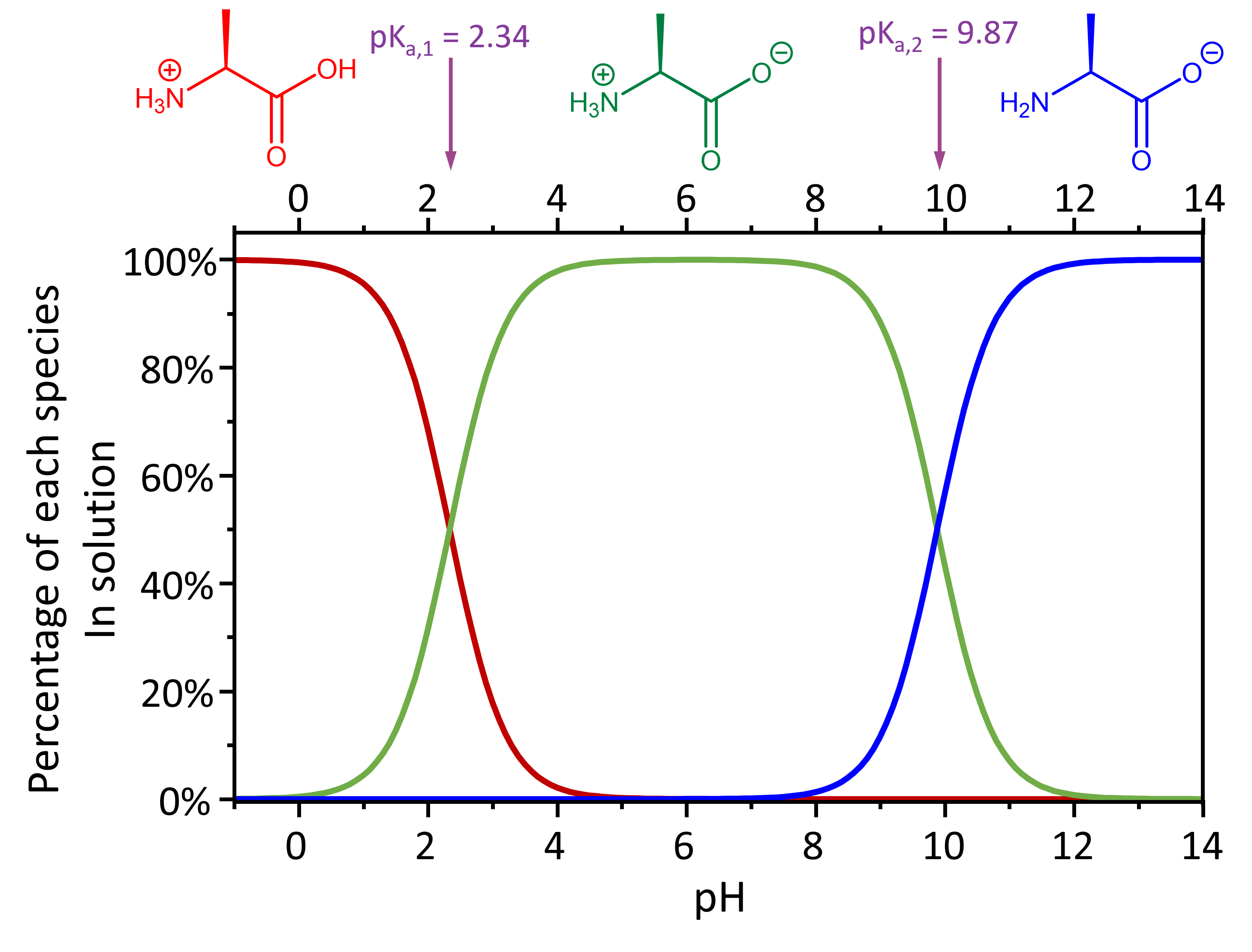
Therefore, at pH < 2.34, the dominant species present in solution is the fully protonated alanine (with an overall +1 charge). At pH > 9.87, the dominant species present is the fully deprotonated alanine (with an overall -1 charge).
In the intermediate pH range (2.34 < pH < 9.87), the dominant form of alanine has an overall neutral charge, but there are two possible structures here: carboxylic acid deprotonated and amine group protonated vs. carboxylic acid protonated and amine group deprotonated. Which structure is it? You can use the methods developed in the preceding section to calculate whether each group is protonated or deprotonated at a given pH.
Exercise: Amino Acid Protonation in Neutral Solution
Based on your calculations, at the pH of a typical living organism, an amino acid exists as a zwitterion (German for “double ion”), a species with no overall electrical charge but with separate parts that are positively and negatively charged.
The formation of a zwitterion is analogous to the acid-base reaction between methylamine (Kb = 4.4 × 10-4) and acetic acid (Ka = 1.8 × 10-5):
where the equilibrium strongly favors products because:
Side Chain Acidity
The backbone amine and carboxylic acid groups undergo condensation reactions to form amide linkages when amino acids polymerize to form proteins. Therefore, in a strand of protein, the side chain “R” group of each amino acid branches off the protein backbone and can influence protein 3D structure. Knowing the nature of these side chains is important for understanding protein 3D structure.
We have previously grouped the twenty amino acids found in human proteins into two groups—nonpolar/hydrophobic vs. polar/hydrophilic—depending on the nature of their side chain. Now, we can further divide the latter group into three groups: polar but neutral, basic, or acidic.
Polar side chain
The six amino acids shown in below have side chains that are polar, but neither acidic nor basic.
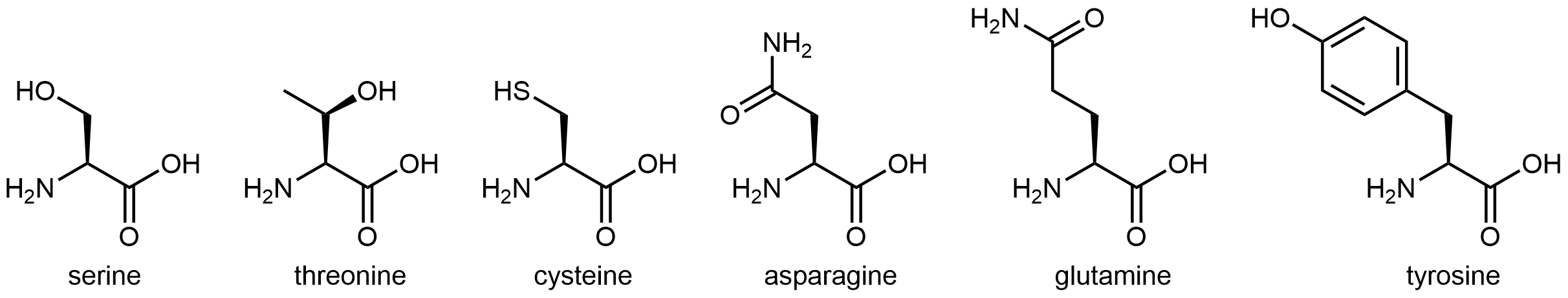
All of these amino acids have a side chain that contains a functional group with significant dipole moment. Five of them can also form strong hydrogen-bonding interactions with water molecules (containing either an alcohol group or an amide group). These characteristics make these side chains hydrophilic, but neither the alcohol group nor the amide group are particularly acidic or basic.
Cysteine has a thiol (-SH) functional group. While it does not form strong hydrogen bonds with water molecules, it does impart a dipole moment to this relatively small side chain, making this side chain overall polar. The thiol functional group is more acidic than the alcohol functional group, but its Ka is still fairly low, ~10-10.
Basic side chain
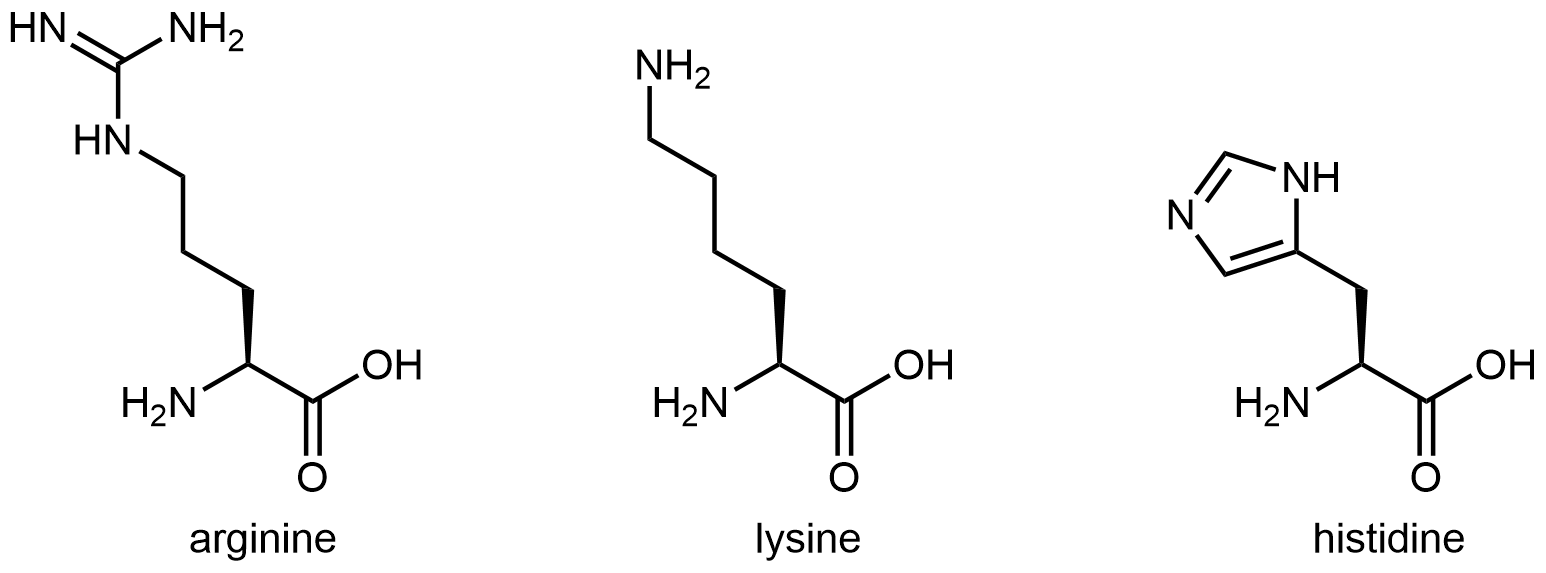
The three amino acids below have side chains containing functional groups that are basic:
Activity: What Part of the Histidine Side Chain Is Most Basic?
Consider the amino acid histidine, whose structure is shown below with each N atom numbered.
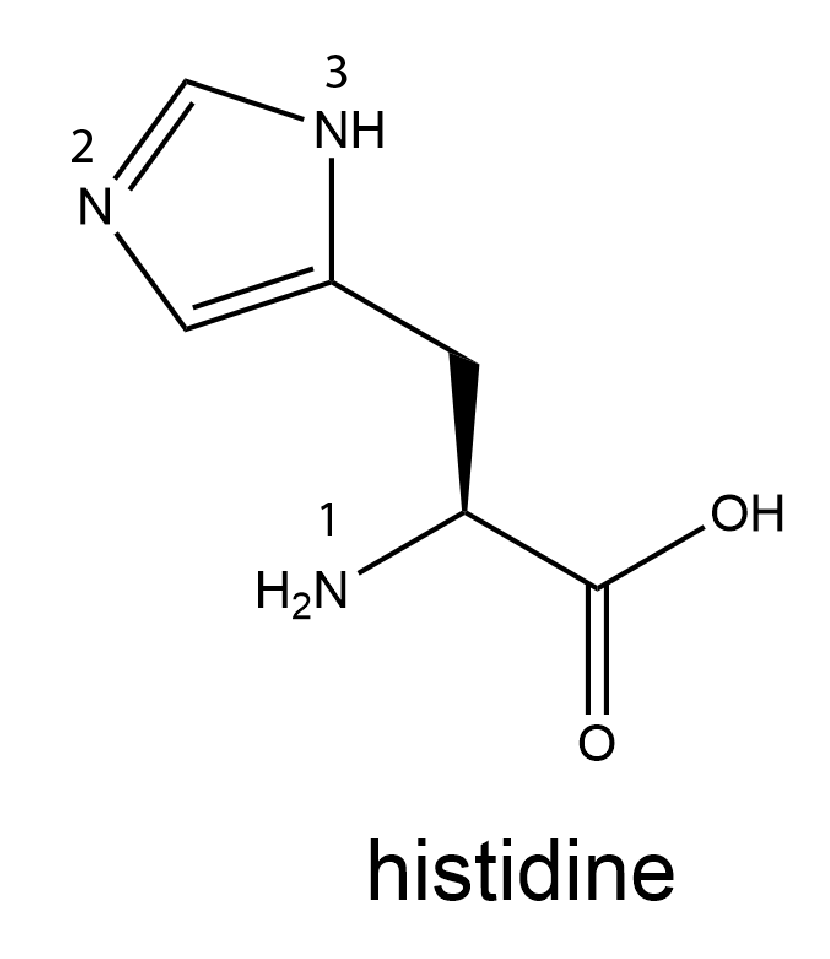
Use the valence bond theory model to analyze the bonding for each N atom in histidine. Write in your notebook the hybridization of each N atom and decide whether the lone pair on each N is in a hybrid orbital or a p orbital. Use your analysis to decide which N atom in the side chain (N-2 or N-3) is more important in making the side chain basic. Write a rationale for your choice. (You may find it helpful to draw a set of resonance structures for the cyclic part of the side chain.)
Write in your notebook, then left-click here for an explanation.
There are three N atoms in histidine. They are numbered in the structure.
N-1 is in the amine group in the amino acid. It is bonded to a C atom and two H atoms and it has a lone pair of electrons. This N atom is sp3 hybridized. (When this N undergoes condensation reaction during protein formation, it becomes part of an amide bond where the hybridization changes to sp2.)
N-2 is in the five-member ring. It is bonded to one C atom with a double bond and to another C atom with a single bond; it has a lone pair of electrons. Because it forms two sigma bonds and has a lone pair, this N atom is sp2 hybridized. The lone pair is in an sp2 hybrid orbital and does not participate in π bonding in resonance structures.
N-3 is in the five-member ring. It is bonded to an H atom and single bonded to two C atoms; it has a lone pair of electrons. Because it forms three sigma bonds and has a double bond in some of the resonance structures, this N atom is sp2 hybridized. The lone pair is in an atomic p orbital and participates in π bonding in resonance structures.
In the discussion of amides, we pointed out that, because the N atom lone pair of electrons is in a p atomic orbital and participates in π bonding in resonance structures, an amide group is much, much less basic than an amine group. The same reasoning applies here. Because the lone pair on N-2 is in an sp2 hybrid orbital and does not participate in π bonding in resonance structures, N-2 is more basic than N-3, and N-2 is more important in making the histidine side chain basic.
The amine functional group at the end of the side chain in lysine imparts basic characteristics to that side chain. The functional group in arginine’s side chain is more complex, but similar to the analysis in the activity above for histidine, you can determine which nitrogen is more important in making the side chain basic by drawing and analyzing a set of resonance structures.
Acidic side chain
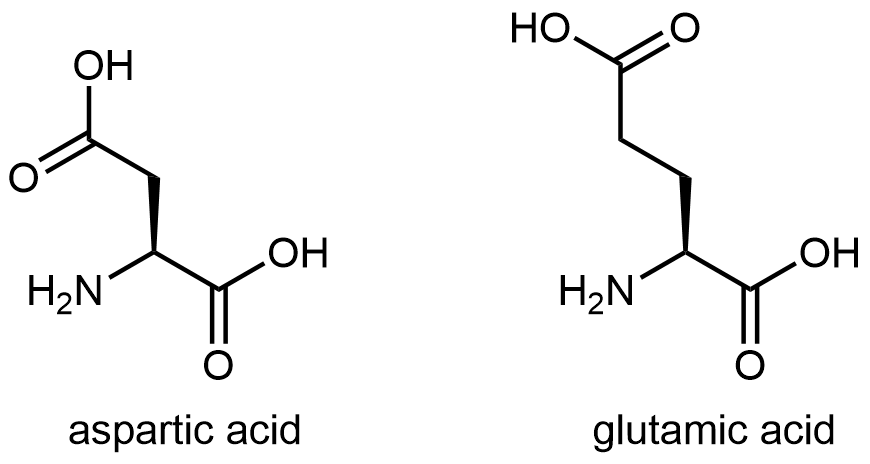
The figure below shows two amino acids that have an acidic side chain containing the carboxylic acid functional group:
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

