D37.2 Predicting the Extent of an Acid Base Reaction
In the previous section, we considered the extent of an acid-base reaction qualitatively: we can predict whether an acid-base reaction is reactant- or product-favored by considering which side has the weaker acid and base.
Specifically, the reaction
is product-favored because there is a weak acid and a strong base on the reactant side and only a weak base on the product side. In other words, the presence of the reactive hydroxide base raises the overall energy of the reactants relative to the products.
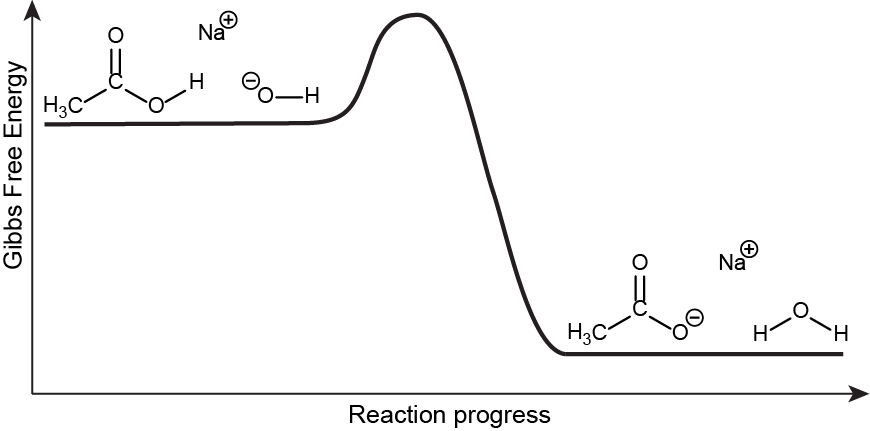
In more quantitative terms, the equilibrium constant of this reaction can be calculated from the known Ka and Kb values. To simplify things a little, let's use the net ionic reaction equation:
we can obtain this reaction equation by combining the reaction equations associated with Ka of acetic acid and Kw (Hess's law):
| CH3COOH(aq) + |
⇌ | CH3COO–(aq) + |
K1 = Ka = 1.8 × 10-5 | |
| ⇌ | K2 = [latex]\dfrac{1}{K_w}[/latex] = 1.0 × 1014 | |||
| Sum: | CH3COOH(aq) + OH–(aq) | ⇌ |
Therefore, the overall reaction equilibrium constant is
which shows that this reaction is indeed product-favored.
What if we instead mix acetic acid with ammonia, a weak base? Is the reaction
reactant-favored or product-favored?
Qualitatively, there is a weak acid (CH3COOH) and a weak base (NH3) on the reactant-side, and a weak acid (NH4+) and a weak base (CH3COO¯) on the product-side. The prediction is not straightforward, so we need to know their Ka and Kb values.
| CH3COOH(aq) | + | NH3(aq) | ⇌ | NH4+(aq) | + | CH3COO¯(aq) |
| Ka = 1.75 × 10-5 | Kb = 1.77 × 10-5 | Ka = 5.65 × 10-10 | Kb = 5.71 × 10-10 |
Now, we can see that CH3COOH is a stronger acid than NH4+, and NH3 is a stronger base than CH3COO¯. The weaker (and therefore more stable) acid and base are both on the product-side, therefore, this reaction is product-favored.
Quantitatively, we can again apply Hess's law to calculated the K for this reaction using the known Ka and Kb values.
| CH3COOH(aq) + |
⇌ | CH3COO–(aq) + |
K1 = Ka, CH3COOH = 1.75 × 10-5 | |
| NH3(aq) + |
⇌ | NH4+(aq) + |
K2 = [latex]\dfrac{1}{K_{a, NH_4^+}}[/latex] = 1.77 × 109 | |
| Sum: | CH3COOH(aq) + NH3(aq) | ⇌ | K = K1K2 = 3.10 × 104 |
The overall reaction equilibrium constant agrees with the qualitative analysis that this reaction is product-favored.
Exercise: Acid-Base Reactions
Left-click here for optional information: application to amphiprotic species
Acid-base reactions can occur between two amphiprotic species, where both reactants can act as either an acid or a base.
For example, two reactions are possible when mixing together a sodium bisulfate solution (NaHSO4(aq), which contains the HSO4- ion) and a sodium bicarbonate solution (NaHCO3(aq), which contains the HCO3- ion):
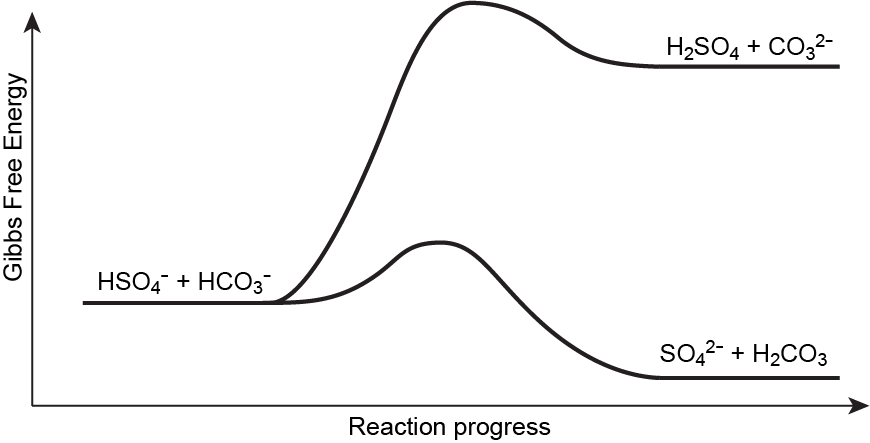
possibility I: HSO4-(aq) + HCO3-(aq) ⇌ SO42-(aq) + H2CO3(aq)
possibility II: HSO4-(aq) + HCO3-(aq) ⇌ H2SO4(aq) + CO32-(aq)
In possibility I, the acids are HSO4- (Ka = 1.1 × 10-2) and H2CO3 (Ka = 4.3 × 10-7) and the bases are HCO3- (Kb = 2.3 × 10-8) and SO42− (Kb = 9.1 × 10-13). While all the species are weak acids and bases, the stronger acid and the stronger base are on the reactant-side. Therefore, this reaction is product-favored at equilibrium.
In possibility II, there is a weak acid and a weak base on the reactant-side, and a strong acid (H2SO4) and a weak base on the product-side. The presence of the strong acid would drive the reaction to be reactant-favored. (the exact values are: the acids: H2SO4 (Ka = 4.0 × 103) and HCO3- (Ka = 4.7 × 10-11), the bases: HSO4- (Kb = 2.5 × 10-18) and CO32− (Kb = 2.1 × 10-4).)
We can also make use of the known Ka values to quantitatively determine which reaction occurs. In possibility I,
| HSO4-(aq) + H2O(ℓ) | ⇌ | SO42-(aq) + H3O+(aq) | K1 = Ka, HSO4¯ = 1.1 × 10-2 | |
| HCO3-(aq) + H3O+(aq) | ⇌ | H2CO3(aq) + H2O(ℓ) | K2 = [latex]\dfrac{1}{K_{a, H_2CO_3}}[/latex] = 2.3 × 106 | |
| Sum: | HSO4-(aq) + HCO3-(aq) | ⇌ | K = K1K2 = 2.6 × 104 |
In possibility II,
| HSO4-(aq) + H3O+(aq) | ⇌ | H2SO4(aq) + H2O(ℓ) | K1 = [latex]\dfrac{1}{K_{a, H_2SO_4}}[/latex] = 2.5 × 10-4 | |
| HCO3-(aq) + H2O(ℓ) | ⇌ | CO32-(aq) + H3O+(aq) | K2 = Ka, HCO3¯ = 4.7 × 10-11 | |
| Sum: | HSO4-(aq) + HCO3-(aq) | ⇌ | K = K1K2 = 1.2 × 10-14 |
Kpossibility I >> Kpossibility II, therefore, the reaction that occurs (proceeds forward and produces products) is possibility I, where HSO4- acts as an acid and HCO3- acts as a base.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

