D39.2 Condensation Reactions
In a condensation reaction, two molecules join to form a larger molecule and a small molecule side product such as H2O or HCl. We have previously discussed condensation polymers, which are made via condensation reactions.
For example, one way to produce ethyl acetate, an ester, is via a condensation reaction between acetic acid and ethanol in the presence of a strong acid:
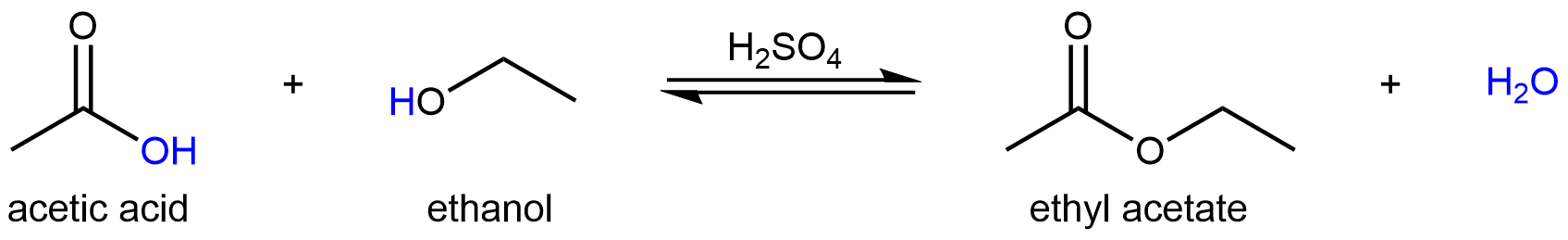
This reaction has a multistep reaction mechanism, and each reaction step is either a Brønsted-Lowry type acid-base reaction or a Lewis type acid-base reaction. So we can apply what we have learned thus far to explore this more complex reaction mechanism.
Of the three starting species in this reaction, sulfuric acid (H2SO4) is a strong acid, acetic acid is a weak acid, and ethanol is not particularly acidic (it is a weaker acid than H2O). When these three compounds are mixed in solution, the initial reactions are Brønsted-Lowry acid-base reactions driven by sulfuric acid:
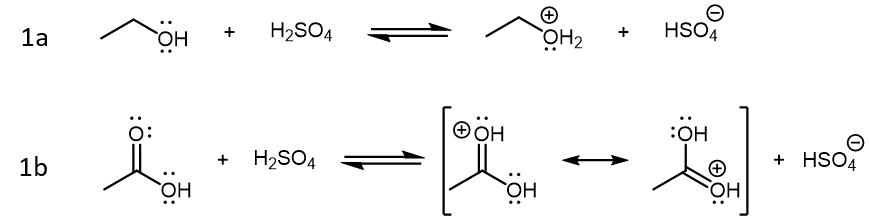
The products formed from these two reactions are conjugate acid of ethanol and acetic acid. Both product acids are strong acids, but protonated ethanol (CH3CH2OH2+) is a little weaker compared to H2SO4 while protonated acetic acid (CH3COOH2+) is stronger compared to H2SO4. Therefore, reaction 1a is product-favored while reaction 1b is reactant favored. However, it is the product of 1b that is the crucial species for step 2 in the reaction mechanism, where this very strong acid acts as a Lewis acid, and an ethanol molecule acts as the Lewis base:
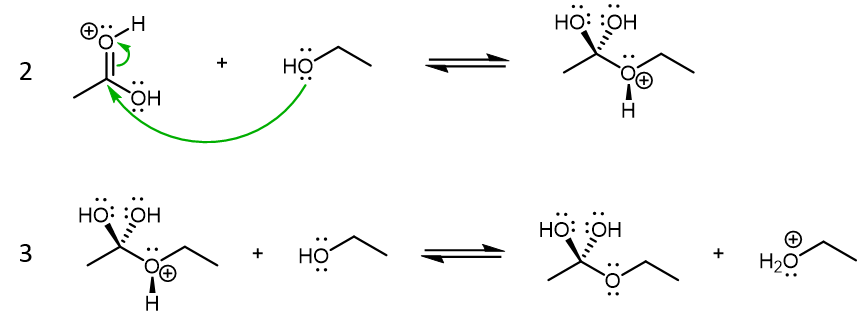
Step 3 is again a Brønsted-Lowry acid-base reaction. The main product here is the molecule with two -OH groups. This molecule will transform into the final ester product.
Steps 4 and 5 will result in one of the -OH groups leaving the molecule. This occurs by protonation of the -OH group via a Brønsted-Lowry acid-base reaction (step 4), followed by the protonated -OH2+ group leaving the molecule as a H2O (step 5):

Reaction 5 is the reverse reaction of a Lewis type acid-base reaction. It results in the formation of a water molecule and a protonated ester.
The last step in the condensation reaction is a final Brønsted-Lowry acid-base reaction that yields the neutral ethyl acetate product:
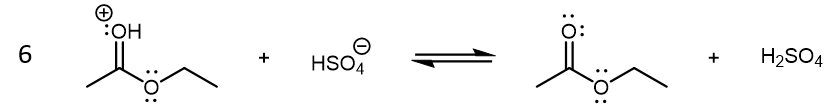
There are several possibilities for reaction 6. For example, an ethanol molecule can be the base instead of HSO4– (ethanol is a stronger base compared to HSO4–.) The specific reaction shown above illustrates the regeneration of H2SO4, which highlights its role as a catalyst.
Each reaction step in this condensation reaction is an acid-base reaction, and each is reversible. This makes the entire condensation reaction reversible. The reverse reaction here is called a hydrolysis reaction (hydrolysis comes from hydro meaning “water” and lysis meaning “breaking apart”). In acid-catalyzed hydrolysis of an ester, a carboxylic acid and an alcohol is formed.
For the condensation reaction we just talked about:

the equilibrium constant for the entire reaction is 3.4, indicating that it is an overall product-favored reaction.
Condensation reactions are a big family of reactions; some occur in acidic solutions and some in basic solutions. The specific reaction steps vary depending on the reactant species and the reaction environment.
Other compounds that can be made via condensation reactions include ethers, which can be obtained from condensation reactions involving two alcohol molecules. If the two alcohol molecules are the same, a symmetric ether forms. For example, when ethanol is heated in the presence of sulfuric acid, diethyl ether and water are formed:
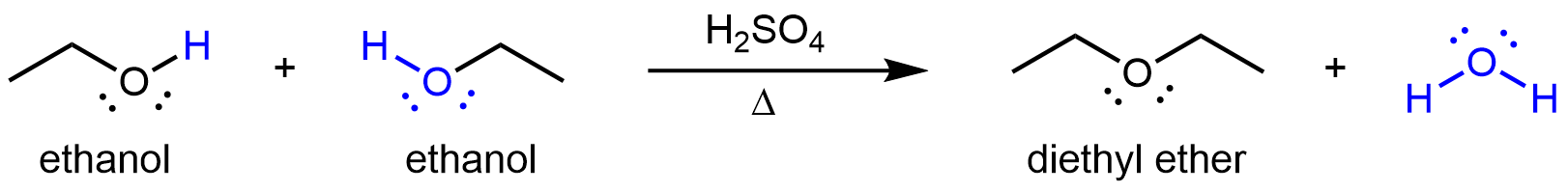
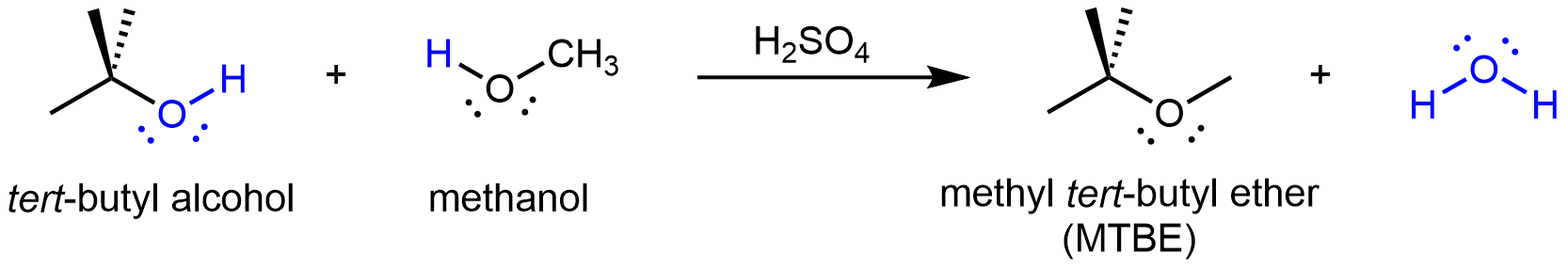
If the condensation reaction involves two different alcohols, an asymmetric ether can form. For example:
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

