D38.4 Acid-Base Indicators
Acid-base indicators are substances that change color when [H3O+] reaches a particular value. Acid-base indicators are either weak organic acids or weak organic bases and can be used to determine the pH of a solution.
Methyl orange is an example of an acid-base indicator. It is a weak acid ("HIn" represents the methyl orange molecule):
If we add an acid to a solution containing methyl orange, the increased [H3O+] will result in more HIn (the red form) at equilibrium. If we add a base, more In- (the yellow form) will be present. The overall color of the solution is the visible result of the ratio of the concentrations of the two species: if most of the indicator is present as In−, then we see the color yellow; if most is present as HIn, then we see the color red.
To consider this more quantitatively, we can rearrange the equation for Ka and write:
When [H3O+] = Ka,HIn, 50% of the indicator is present in the red form (HIn) and 50% is in the yellow ionic form (In-), and the solution overall appears orange in color. When [H3O+] increases to 8 × 10−4 M (pH = 3.1), the solution turns red. No significant change in color is visible for further increase in [H3O+]. When [H3O+] decreases to 4 × 10−5 M (pH = 4.4), most of the indicator is in the yellow ionic form, and further decrease in [H3O+] does not produce a visible color change. The pH range of 3.1 - 4.4 is the color-change interval of methyl orange, the range of pH values over which the color change takes place.
There are many different acid-base indicators. Their color-change intervals have a wide range of pH values (see figure below). Universal indicators and pH paper contain a mixture of indicators and exhibit different colors at different pH values.
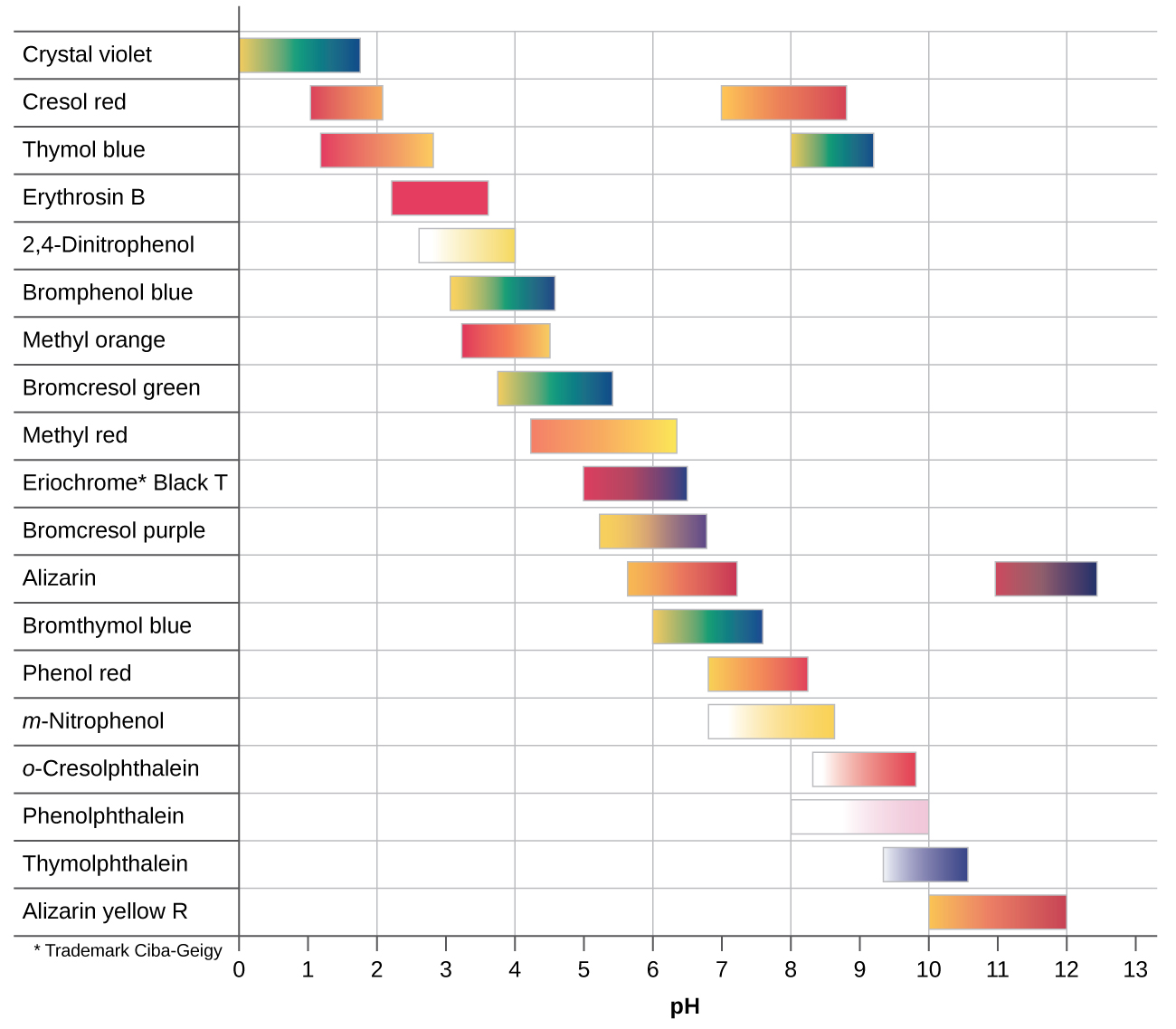
The best indicator for a titration would have a color-change at the equivalence point of the titration.
For example, the figure below overlays the color-change intervals of three indicators on top of the titration curves of HCl and CH3COOH. The steep section of both titration curves spans the color-change interval of phenolphthalein. We can use phenolphthalein for titrations of either acid.
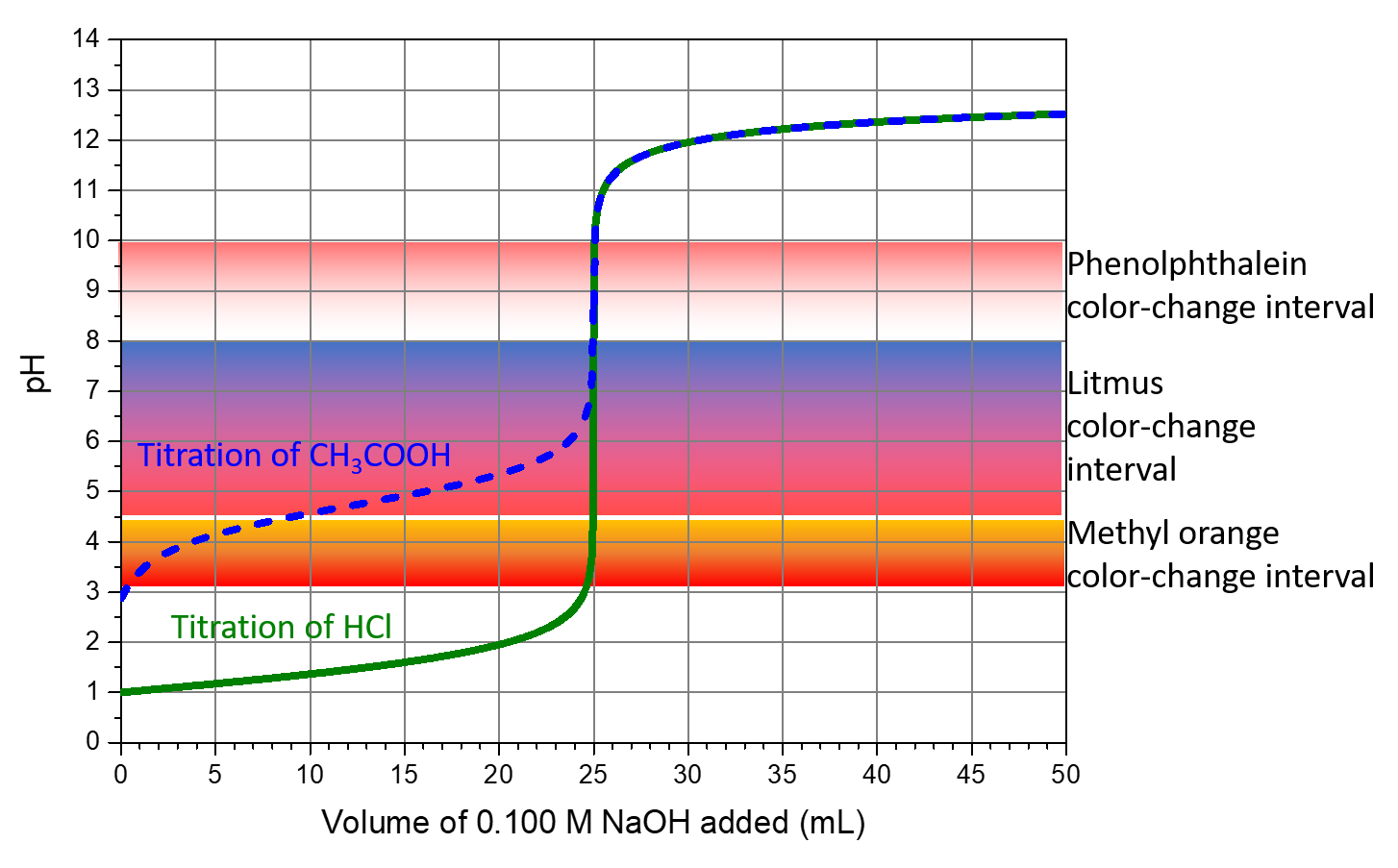
Litmus is a suitable indicator for the HCl titration. However, we should not use it for the CH3COOH titration. This is because the solution pH is within the color-change interval when only ~8 mL of NaOH has been added, well before the equivalence point. The color change would be very gradual, taking place during the addition of ~17 mL of NaOH, making litmus inaccurate for this titration.
We may use methyl orange for the HCl titration, but it would not give very accurate results: (1) It completes its color change slightly before the equivalence point is reached (but very close to it, so this is not too serious); (2) it changes color during the addition of 0.5 mL of NaOH, which is not so sharp a color change as that of litmus or phenolphthalein. Methyl orange would be completely unsuitable as an indicator for the CH3COOH titration. Its color change is completed long before the equivalence point is reached and hence provides no indication of the equivalence point.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

