D34.1 Acid-Base Reactions
We are now in a position to apply knowledge developed in earlier units to explore an important and very common class of chemical reactions: acid-base reactions. We will discuss the definition of what is an “acid” or “base” in the next section, but first let us consider some general ideas involved in acid-base reactions.
While acid-base reactions can occur in a variety of solvents, in this course, unless otherwise noted, the solvent is water. In other words, the reaction occurs in an aqueous solution. An H2O molecule is polar and can form hydrogen-bonds. As we will see later, an acid or base molecule is also polar and can form hydrogen-bonds. This means that, generally speaking, acids and bases have fairly high solubility in water.
In addition to acting as the solvent, H2O can also actively participate in an acid-base reaction—it can react as an acid or a base with the substance dissolved in it—it is both the solvent and one of the reactants! As we previously discussed, an important aspect of hydrogen-bonding is the partial electron sharing between the hydrogen-bonding partners, which resembles the beginning of the formation of a covalent bond. Hence, when water interacts with the dissolved acid or base via dipole-dipole and hydrogen-bonding interactions, it is possible for an acid-base reaction to proceed.
For example, when acetic acid (CH3COOH, an acid) is dissolved in water, it can form several hydrogen bonds with water molecules.
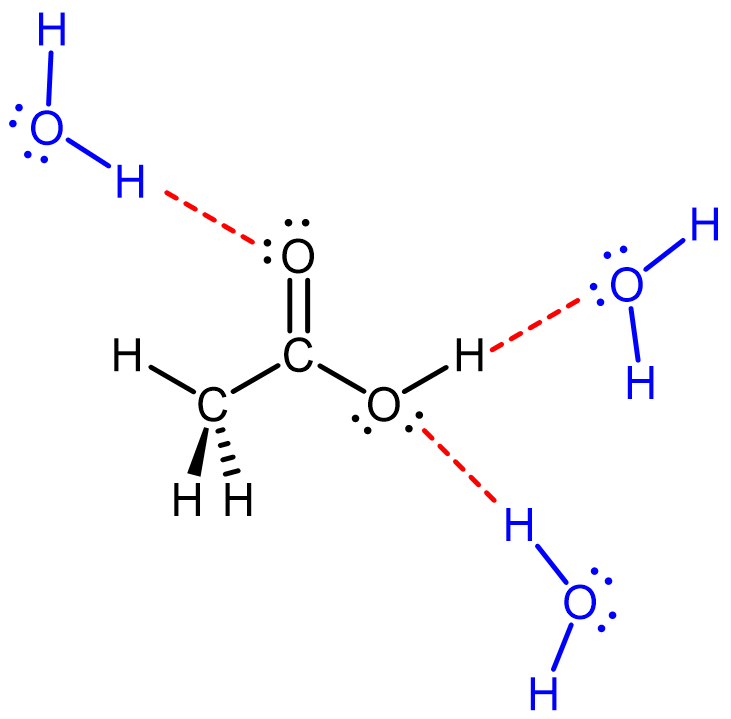
The most notable interaction is the one between the electron-deficient δ+ H in O-H in acetic acid with the electron-rich δ¯ O in H2O:
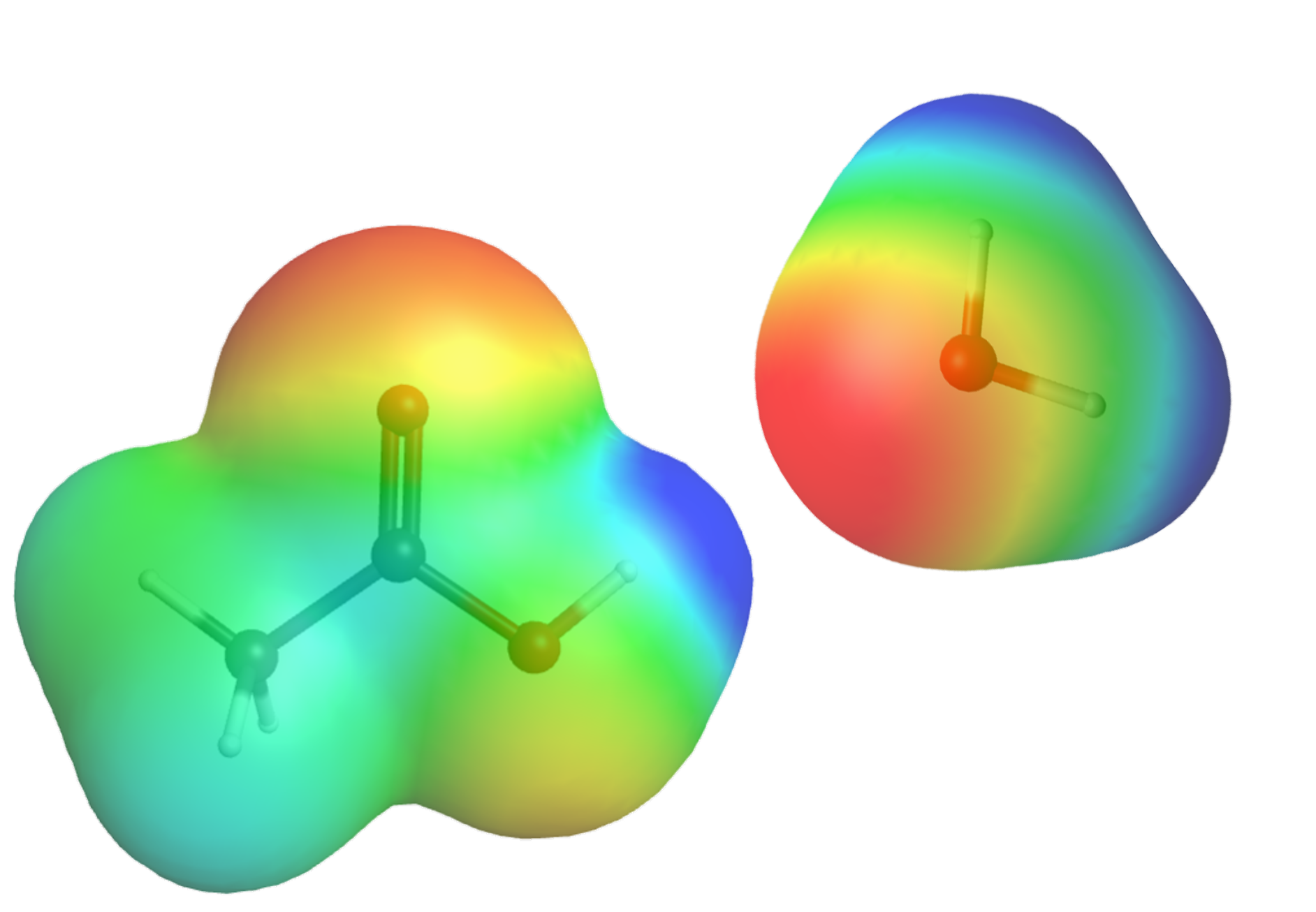
which allows electron transfer from the fully-occupied lone pair orbital in H2O to the empty O-H anti-bonding σ* orbital in CH3COOH, leading to breaking of the O-H bond in CH3COOH (CH3COOH loses an H+) and forming of a new O-H in H2O (H2O gains an H+).
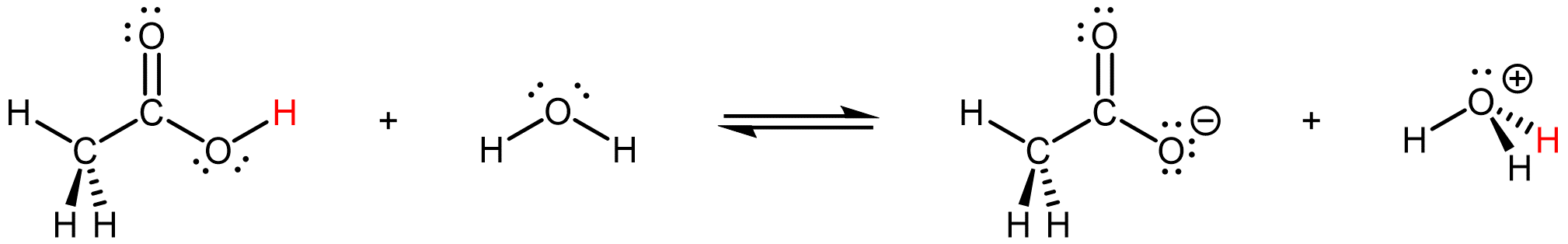
In this reaction, CH3COOH acts as the acidic species while H2O acts as the basic species while also serving as the solvent in which the reaction occurs. (Note that the other hydrogen-bonding interactions between CH3COOH and H2O has similar types of orbital overlaps that can potentially lead to reactions where CH3COOH acts as a base instead of an acid. However, the ΔG of those reactions are less favorable than the one above, and therefore the acid-base reaction observed when acetic acid is dissolved in water is the one above.)
Let’s consider another example, where ammonia is dissolved in water. Again, δ+ and δ¯ sites on NH3 interact with those on H2O, making it miscible in water.
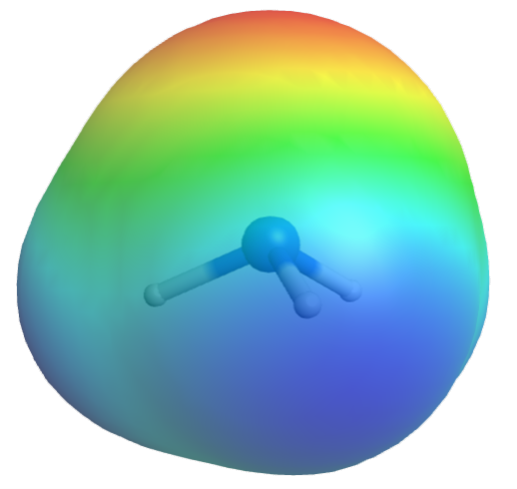
Here, one of the NH3-H2O interactions is between the electron-rich δ¯ N in NH3 and the electron-deficient δ+ H in H2O, which allows electron transfer from the occupied lone pair orbital in NH3 to one of the empty O-H anti-bonding σ* orbital in H2O.
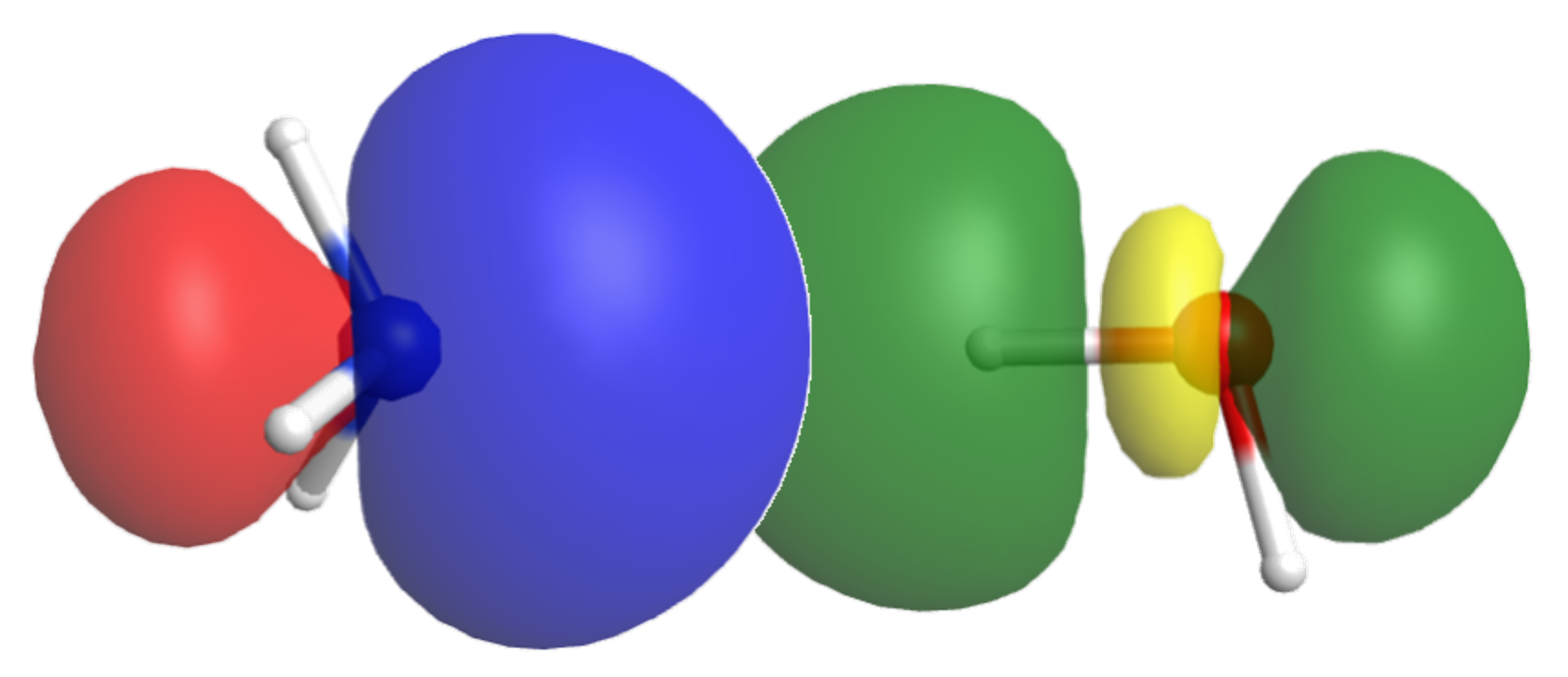 The resulting acid-base reaction is:
The resulting acid-base reaction is:
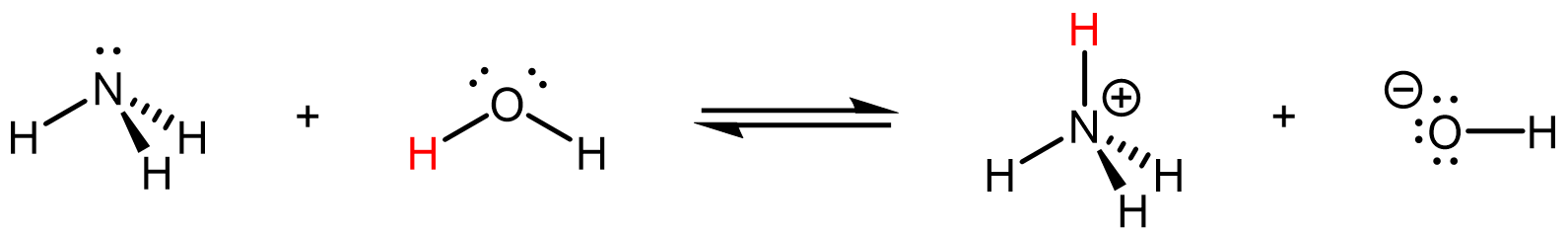 where NH3 acts as the basic species while H2O acts as the acidic species.
where NH3 acts as the basic species while H2O acts as the acidic species.
Because water can react as an acid or a base, having acid-base reactions occurring in an aqueous environment has some consequences, which we will discuss in more detail as we delve deeper into this class of reaction.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

