D37.1 Acid-Base Reactions
Thus far, we have considered acid-base chemistry in terms of either an acid or a base reacting with water in aqueous solutions. Let us take it one step further and consider reacting acids with bases to make new substances, most often in aqueous solutions.
Like any reaction, thermodynamics dictates that the side with lower energy/more stable species (the side with the weaker acid and base) is favored at equilibrium. In other words, if the weaker acid and weaker base are on the reactant side, the reaction is reactant-favored at equilibrium; if the weaker acid and weaker base are on the product side, the reaction is product-favored at equilibrium. The strengths of acids and bases can be compared by their Ka and Kb values listed in a reference table.
For example, when hydrochloric acid and sodium hydroxide are mixed together, we have an acid-base reaction:
On the reactant side, HCl is a strong acid (Ka > 1) that reacts completely in water—the HCl(aq) solution contains only H3O+(aq) and Cl¯(aq), and the solution pH is <7. NaOH is a strong base—it is an ionic compound consisting of Na+ and OH- with high solubility in water—the NaOH(aq) solution contains only Na+(aq) and OH¯(aq), and the solution pH is >7.
On the product side, there are Na+(aq), Cl−(aq), and H2O(ℓ). Cl−is the conjugate base of a strong acid, it has negligible base strength and does not react as a base. H2O is the conjugate acid of a strong base (OH¯), it has negligible acid strength (Kw << 1). Na+ cannot be a Bronsted-Lowry acid or base—it has no H+ to donate and, being a small positively charged ion, cannot accept a H+ either. These three products in solution gives a pH = 7.
This reaction, with strong acid and base on the reactant side and none/extremely-weak acid and base on the product side, has an equilibrium that heavily favors the product side. The net ionic reaction is
which has an equilibrium constant of [latex]K = \dfrac{1}{K_w} = 1.0 \times 10^{14}[/latex] at 25 °C.
Note that one of the products is an ionic compound. Whether this product precipitates out of the solution or not depends on its solubility. In this case, NaCl is a very soluble salt, so it remains in solution.
Let us consider another example where acetic acid is mixed with sodium hydroxide:
On the reactant side, there is a weak acid and a strong base. On the product side, there is a weak base (CH3COO¯). Therefore, this reaction equilibrium favors the product side. In other words, this reaction is driven forward by the reactivity of the strong base.
CH3COO-, the conjugate base of acetic acid, is a weak base. Its presence in the final solution results in a pH > 7, dictated by the acetate anion's reaction in water:
which correspond to its Kb. (Some reference tables only report dissociation constants for acids; Kb can be calculated as Kw/Ka of the conjugate acid (acetic acid in this case).)
Exercise: Relationship between Dissociation Constants of an Acid and Its Conjugate Base
Molecular compounds often have properties that hinder their pharmaceutical usage, such as those related to solubility, dissolution rate, bioavailability, and chemical/physical stability. One way to address this is to convert them into an ionic compound, and as such, nearly half of the small molecule drugs are commercialized as salts.
An example of this is diphenhydramine (DPH), which contains an amine functional group, making it a weak base.
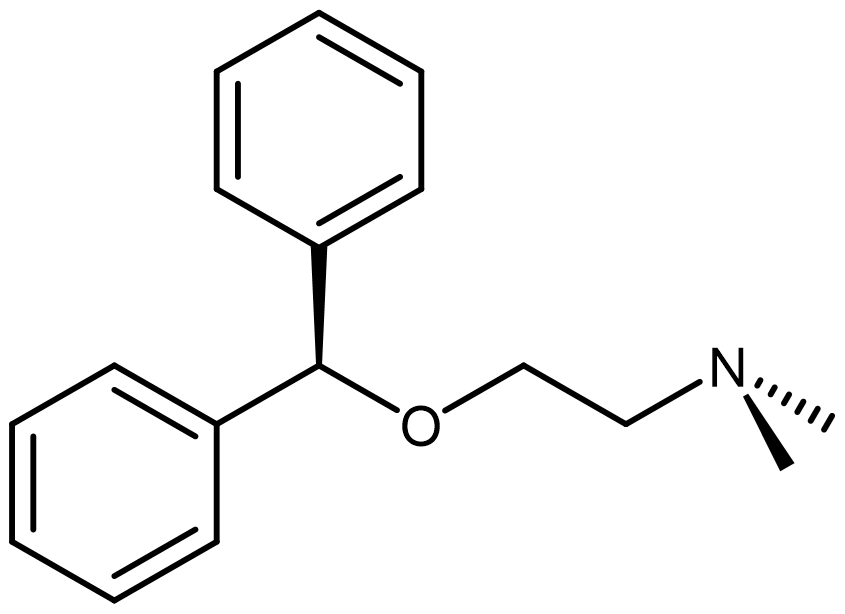
It is often available as "diphenhydramine HCl":

When HCl is added to DPH, an acid-base reaction occurs where the amine group in DPH is protonated. The Cl¯ that is left serves as the counterion to the organic cation, forming a crystalline product.
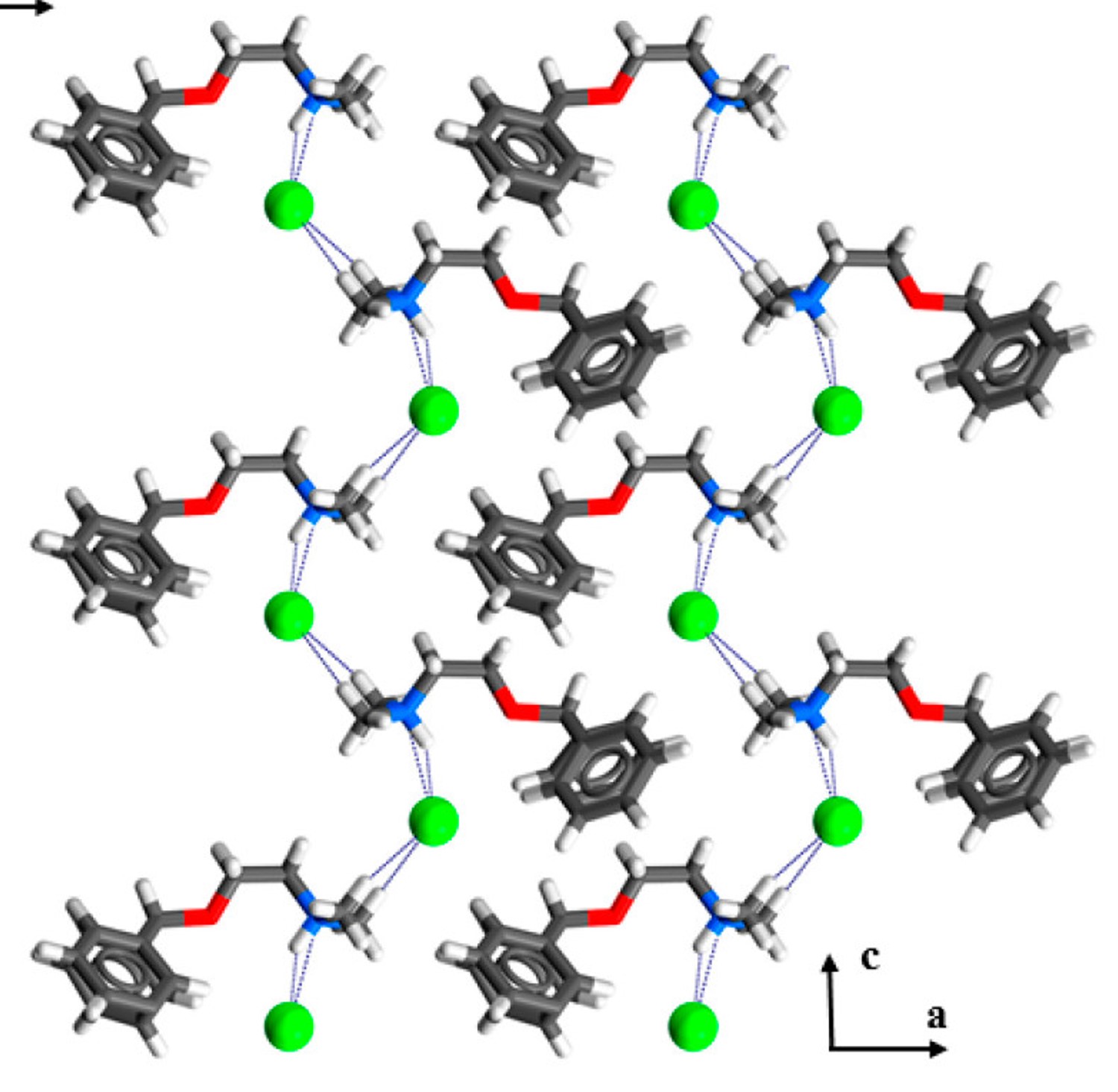
Similar to the acetic acid and sodium hydroxide reaction above, the reactivity of the strong acid HCl drives the reaction equilibrium to favor the product side.
Activity: pH of an Ammonium Salt
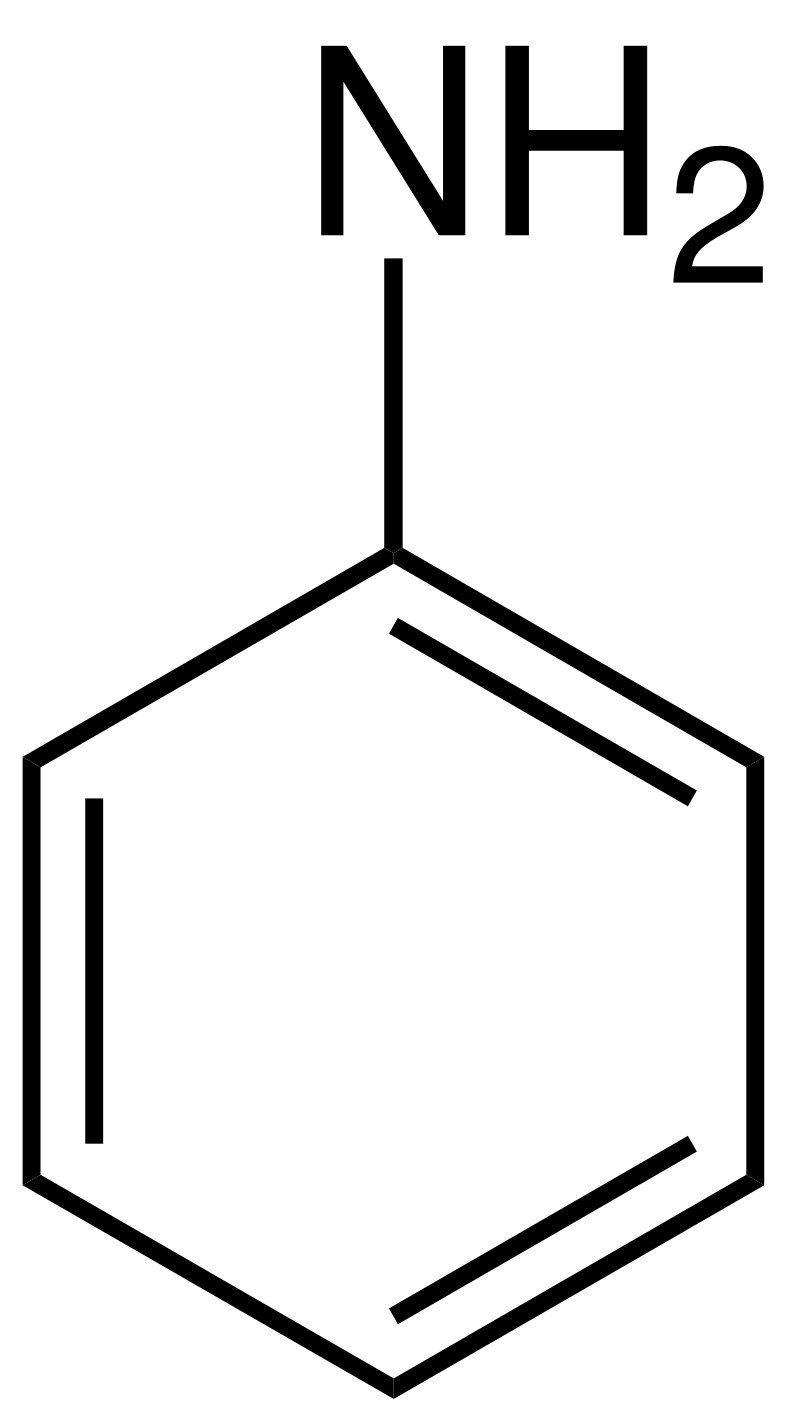
Aniline (C6H5NH2, Kb = 3.9 × 10−10 at 25 °C) is an amine that is used to manufacture dyes. It is isolated as aniline hydrochloride, [C6H5NH3]Cl, a salt prepared by the reaction of the weak base aniline and hydrochloric acid. Calculate the pH of a 0.233-M solution of aniline hydrochloride at 25 °C. The relevant reaction that is affecting the solution pH is:
C6H5NH3+(aq) + H2O(ℓ) ⇌ C6H5NH2(aq) + H3O+(aq)
As the examples above show, the reactivity of a strong acid or a strong base is often leveraged to drive a reaction to form the desired product. The commonly used strong acids are:
| HClO4 | Perchloric acid |
| HI | Hydroiodic acid |
| HBr | Hydrobromic acid |
| HCl | Hydrochloric acid |
| H2SO4 | Sulfuric acid |
| HNO3 | Nitric acid |
Their conjugate base have negligible basicity in aqueous solutions.
The commonly used strong bases are:
| LiOH | Lithium hydroxide |
| NaOH | Sodium hydroxide |
| KOH | Potassium hydroxide |
| Ca(OH)2 | Calcium hydroxide |
| Sr(OH)2 | Strontium hydroxide |
| Ba(OH)2 | Barium hydroxide |
which are all ionic compounds containing the hydroxide anion.
Exercise: Is a Solution Acidic or Basic?
Just as you can add hydroxide to a solution in the form of the NaOH salt, thereby raising the solution pH, aqueous solutions of other salts may be neutral (pH = 7), acidic (pH < 7) or basic (pH > 7).
Oftentimes, only one of the ions in the salt has an acidity or basicity, such is the case for NaOH. However, sometimes both the cation and anion have some acid-base characteristics. In such a case, we must know the Ka of the acid and the Kb of the base. If Ka > Kb, the solution is acidic; if Kb > Ka, the solution is basic.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

