D34.4 Acid Constant and Base Constant
The relative strengths of Brønsted-Lowry acids and bases can be evaluated by comparing the equilibrium constants for their acid-base reactions with water. For the reaction of a generic acid, HA:
we write the acid reaction equilibrium constant (Ka) expression as:
(Although water is a reactant in the reaction, it is also the solvent with its phase indicated as "ℓ", so we do not include [H2O] in the expression.) Ka is sometimes also referred to as acid dissociation constant or acid ionization constant.
A stronger acid, which dissociates to a greater extent, would have a larger Ka compared to a weaker acid. In other words, compared to an acid with a smaller Ka, an acid with a larger Ka would have a higher concentration of H3O+ and A− relative to the concentration of the non-dissociated acid, HA, at equilibrium.
For example, these data on acid equilibrium constants:
| CH3COOH(aq) + H2O(ℓ) | ⇌ | CH3COO-(aq) + H3O+(aq) | Ka = 1.8 × 10-5 |
| HNO2(aq) + H2O(ℓ) | ⇌ | NO2-(aq) + H3O+(aq) | Ka = 7.4 × 10-4 |
| HSO4-(aq) + H2O(ℓ) | ⇌ | SO42-(aq) + H3O+(aq) | Ka = 1.1 × 10-2 |
indicate that the order of acid strength is: acetic acid (CH3COOH) is a weaker acid than nitrous acid (HNO2) which is a weaker acid than hydrogen sulfate ion (HSO4-).
We can consider the strength of a base (B) similarly by considering the extent that it will form hydroxide ions in an aqueous solution:
where the base equilibrium constant (Kb) expression is:
A stronger base has a larger Kb than a weaker base.
Notice that Ka and Kb provide a quantitative measure of acid and base strengths—significantly more accurate than qualitative descriptions of "strong acid" or "weak acid".
Left-click here for optional information
Try this simulation of strong and weak acids and bases at the molecular level.
A common means of expressing values that span many orders of magnitude, such as those of Ka and Kb, is to use a logarithmic scale. One such scale is based on the p-function:
where “X” is the quantity of interest and “log” is the base-10 logarithm. For example:
| HNO3(aq) + H2O(ℓ) | ⇌ | NO3-(aq) + H3O+(aq) | Ka = 27 | pKa = -1.43 |
| CH3COOH(aq) + H2O(ℓ) | ⇌ | CH3COO-(aq) + H3O+(aq) | Ka = 1.8 × 10-5 | pKa = 4.74 |
| CH3CH2O-(aq) + H2O(ℓ) | ⇌ | CH3CH2OH(aq) + OH¯(aq) | Kb = 79 | pKb = -1.9 |
| NH3(aq) + H2O(ℓ) | ⇌ | NH4+(aq) + OH¯(aq) | Kb = 1.8 × 10-5 | pKb = 4.74 |
Now let us consider the reactions for a conjugate acid base pair, HA and A−:
A-(aq) + H2O(ℓ) ⇌ HA(aq) + OH-(aq) [latex]K_{\text{b}} = \dfrac{[\text{HA}][\text{OH}^{-}]}{[\text{A}^{-}]}[/latex]
Adding these two chemical equations yields the equation for the autoionization of water:
Therefore,
and
For example, at 25 °C, Ka of acetic acid (CH3COOH) is 1.8 × 10−5 M, and Kb of its conjugate base, acetate anion (CH3COO-), is 5.6 × 10−10 M. The product of these two equilibrium constants is indeed equal to Kw:
This relationship means that when you know the Ka of an acid at a given temperature, you also know the Kb of its conjugate base. Therefore, in reference tables, such as the ones in the appendix, often only one (either the Ka of an acid or the Kb of its conjugate base) are listed.
This relationship also tells us that stronger acids form weaker conjugate bases, and weaker acids form stronger conjugate bases.
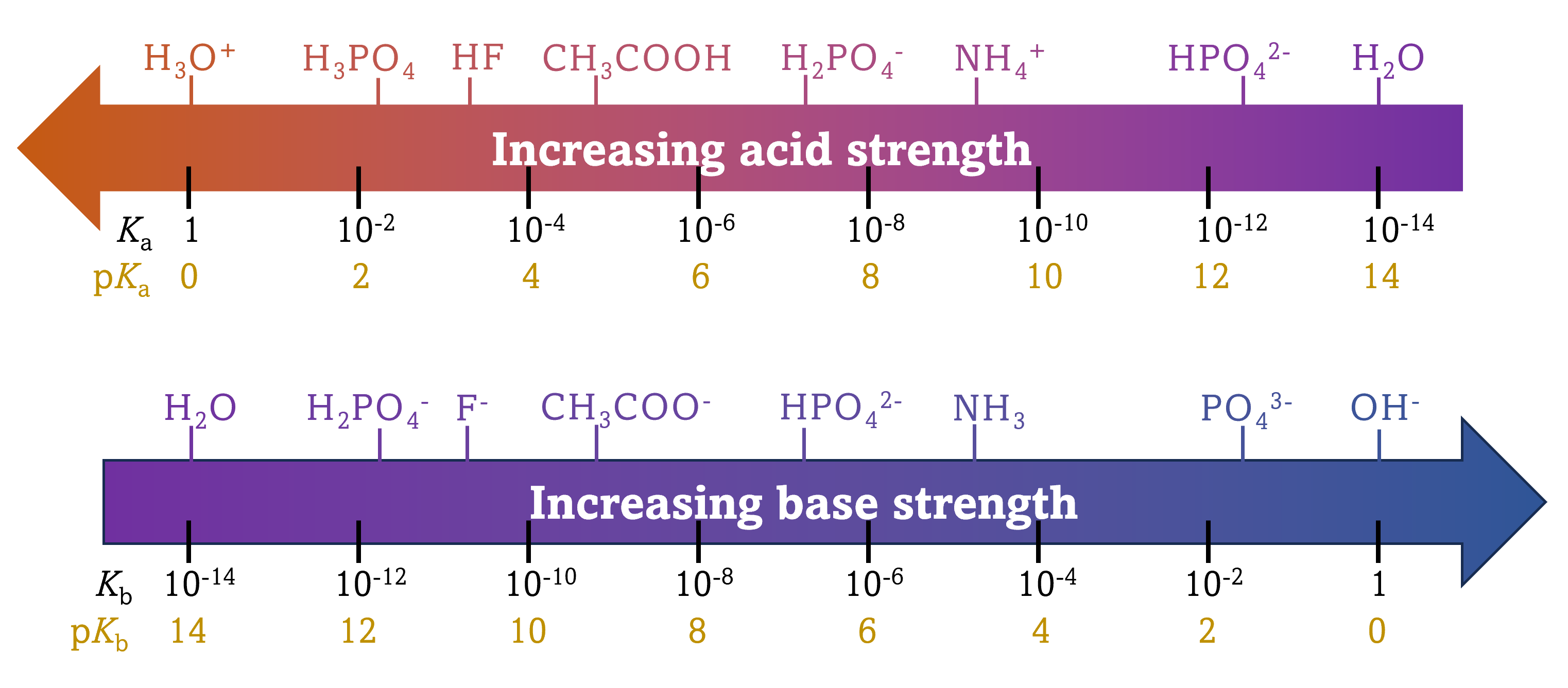
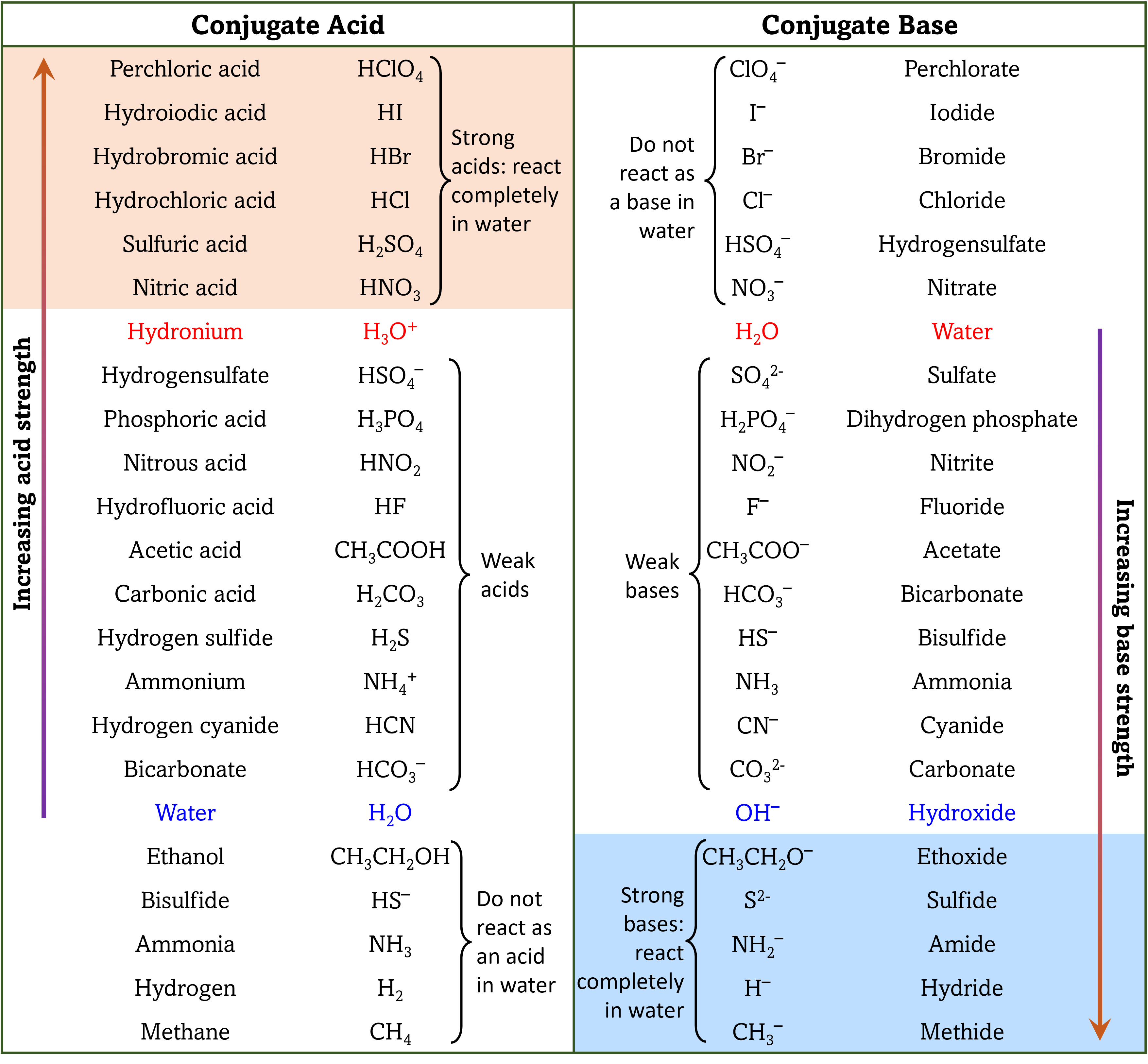
Although "strong" and "weak" are relative terms, we generally refer to acids stronger than H3O+ as strong acids, and those weaker than H3O+ as weak acids. Similarly, bases stronger than OH- are referred to as strong bases while those weaker than OH- are weak bases. Within these broad category of strong vs. weak, any specific acid may be stronger or weaker than another acid. For example, both hydrofluoric acid (HF) and acetic acid (CH3COOH) are weak acids, but HF is a stronger acid than CH3COOH. The same goes for bases.
Because strong acids and strong bases react completely in aqueous solutions, the concentration of the unreacted form is essentially zero. For example, in a 0.10-M solution of HCl, [HCl]e = 0, [H3O+]e = 0.10 M, and [Cl−]e = 0.10 M. A consequence of this complete reaction is that in aqueous solution there is no way to tell whether one strong acid is stronger than another: HCl, HBr, and HI all are completely reacted. This is known as the leveling effect of water. However, when dissolved in other solvents, these acids do not react completely. The extent of reaction increases in the order HCl < HBr < HI, and so HI is the strongest of these acids. Water exerts a similar leveling effect on strong bases.
Many acids and bases are considered "weak". A solution of a weak acid in water is an equilibrium mixture of the acid, hydronium ion, and the conjugate base of the acid.
Exercise: Brønsted-Lowry Acids and Bases
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

