D35.3 Base Strength and Molecular Structure
The same thermodynamic considerations apply when thinking about the basicity of a generic base A– in the equilibrium:
It is the energy of A¯ + H2O relative to HA + OH– that dictates the strength of the base.
The reasoning applied for understanding relative acid strengths also apply here in understanding relative base strengths. For example, acetate is a much weaker base than ethoxide:
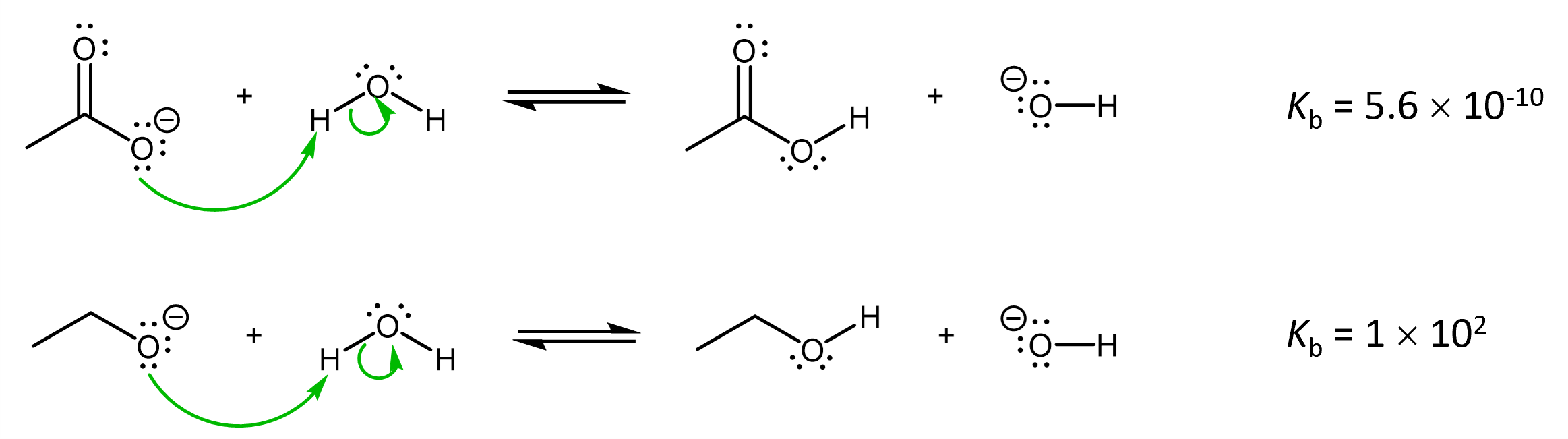
This is because the excess electron density in the acetate anion is stabilized via delocalization over both O atoms, whereas the extra electron in ethoxide is localized on one O atom. This stabilization in acetate anion makes ΔrG° more positive (lowers the reactant energy relative to the product energy), making acetate a weaker, less reactive, base when compared to ethoxide.
When an acid molecule reacts, it loses a H+, and the polarity of the covalent bond involving that H in the acid molecule affects how readily that H+ leaves the molecule. For example, the O-H bond in acetic acid is less polar than the O-H bond in fluoroacetic acid, which contributes to acetic acid being the weaker acid.
There is a similar consideration when thinking about base strength. Namely, the base molecule is donating electrons towards the formation of the new covalent bond as it accepts the H+. Thus, the availability of that electron density influences how basic a molecule is.
For example, let’s compare methanol and methylamine as bases in an acid-base reaction:
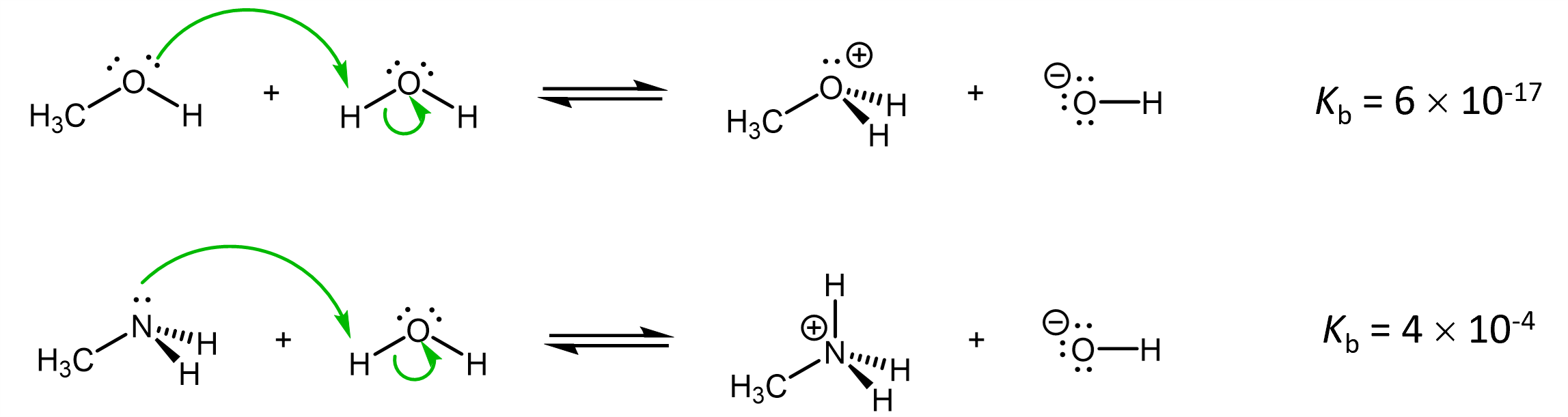
Both the O in methanol and N in methylamine has a lone pair capable of going on to form the O-H and the N-H bond, respectively. However, O is more electronegative than N, and the lone pair in methanol is in a lower energy orbital (more stable) than the lone pair in methylamine. The lower energy lone pair, associated with the more electronegative atom, is less reactive, making methanol a weaker base than methylamine.
Again, similar to the acid strength reasoning, the availability of the lone pair for reaction is influence by electron delocalization. For example, aniline:
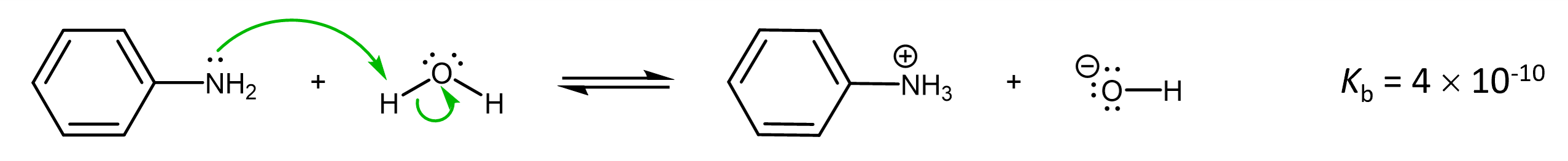 is a weaker base than methylamine, because the lone pair on N in aniline is delocalized:
is a weaker base than methylamine, because the lone pair on N in aniline is delocalized:

resulting in the N in aniline having less electron density than the N in methylamine.
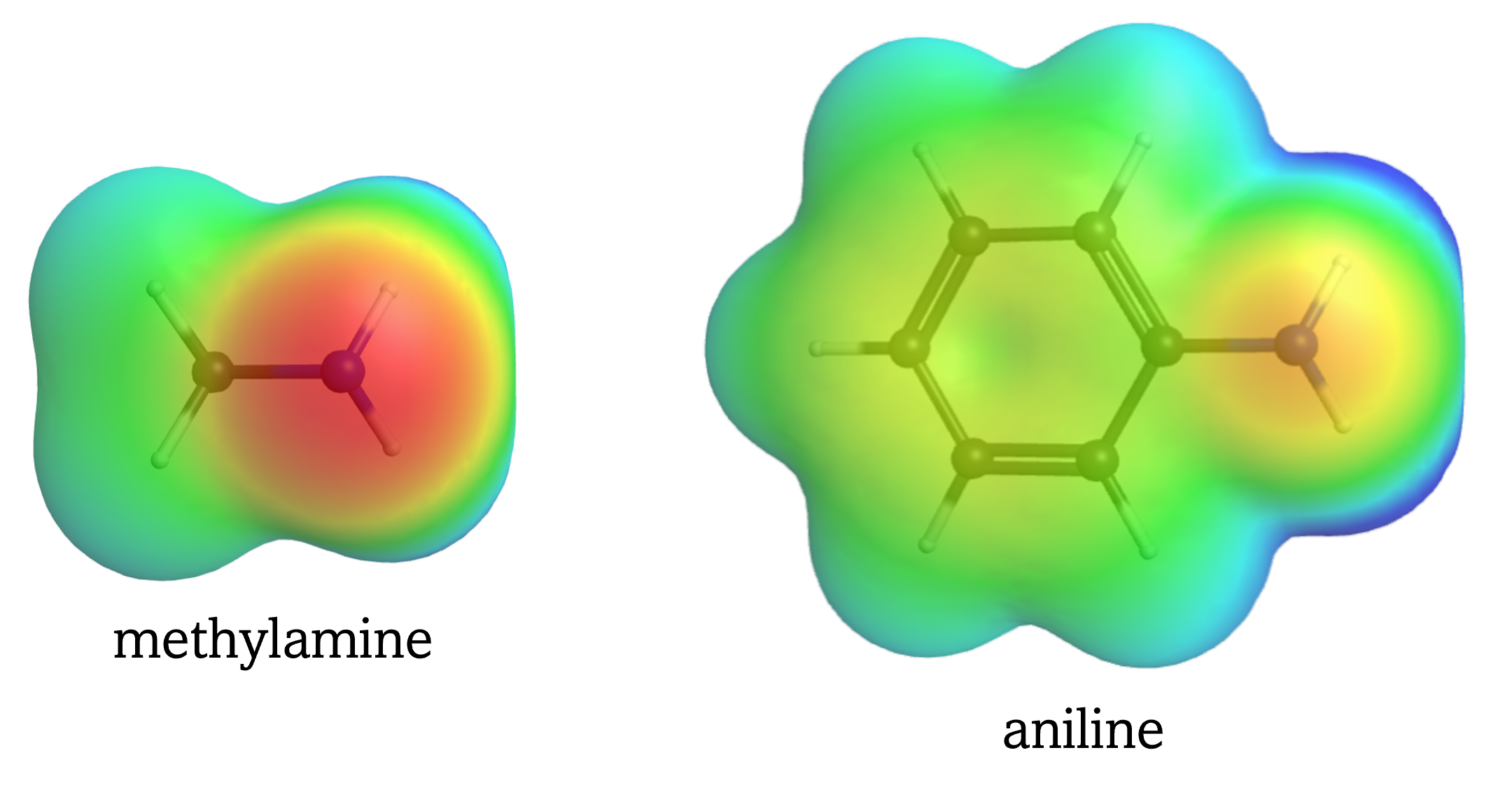
The same reasoning can be applied to the comparison of ethoxide and acetate at the top of the page: both bases have excess electron density, but that electron density is delocalized in the case of acetate, making it less available for reaction.
Activity: Inductive Effect
The presence of substituents that can pull electron density away or push electron density onto the amine functional group via the inductive effect will also influence the basicity of the molecule.
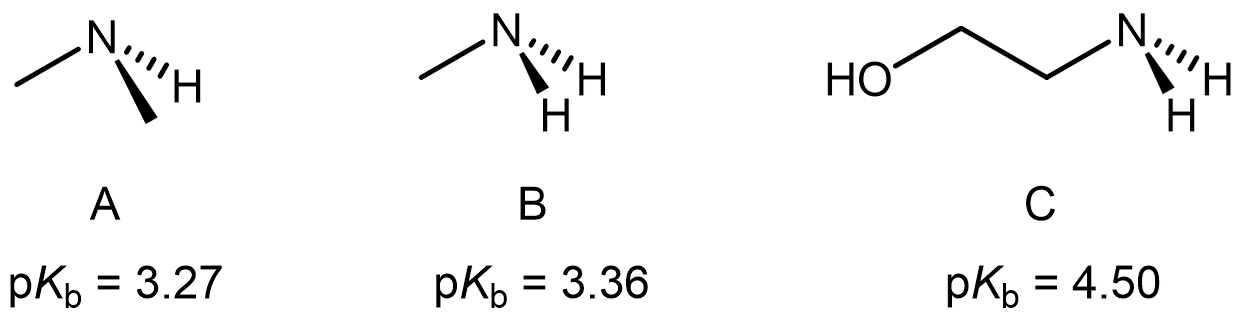
Consider the following three molecules with their observed pKb values. Explain their relative basicity based on their structural differences.
Write in your notebook, then left-click here for an explanation.
The inductive effect from a methyl group would increase the electron density on N. Therefore, molecule A, with two methyl substituents, would have higher base strength than molecule B with only one methyl substituent.

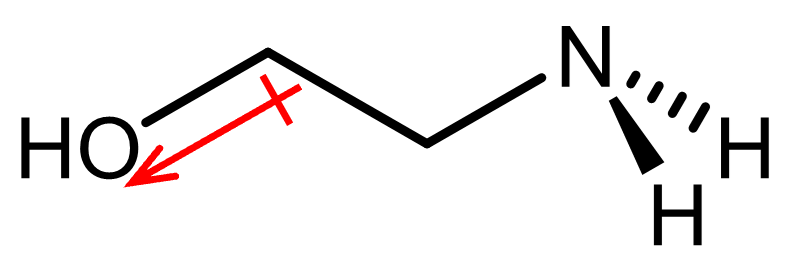
The alcohol group in molecule C would exert an inductive effect that pulls electron density from N, resulting in molecule C having a weaker base strength.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

