D14.6 Carboxylic Acids, Amines, Amides
Carboxylic Acids
A carboxylic acid functional group, -COOH, has a hydroxyl group linked to a carbonyl carbon atom. It differs from an ester in that the non-carbonyl oxygen is bonded to a hydrogen atom rather than an R group. Hence, carboxylic acid groups are found at one end of a molecule.
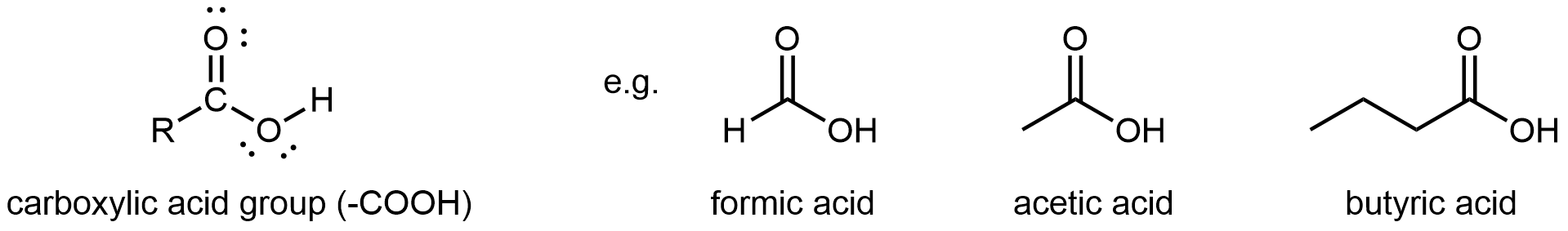
The simplest carboxylic acid is formic acid, known since 1670. Its name comes from the Latin word for ant, formicus. It is partially responsible for the pain and irritation of ants’ and wasps’ stings. Acetic acid is a main component (>4% by volume) of vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present; yeast changes sugar to ethanol, which is then converted to acetic acid via biological oxidation. Butyric acid, a component of rancid butter and Limburger cheese, has a vile odor.
As their name suggests, carboxylic acid groups are acidic. This is because their O-H bond can break relatively easily to yield -COO– and H+, allowing them to donate a proton, H+, to another molecule or ion. This acidity arises from the relative stability of the carboxylate anion, RCOO−.
Activity: Resonance Structures and Acidity of a Carboxylic Acid
Acetic acid (CH3COOH) is acidic because it can ionize to form the acetate anion and a hydrogen ion (proton):
CH3COOH ⟶ CH3COO− + H+
Draw the Lewis structure for acetate ion. Are there resonance structures for acetate anion? Remember that electron delocalization, as depicted in resonance structures, lowers the energy of a molecule or ion (stabilizes the molecue or ion).
Write a statement giving a reason that you agree with, or disagree with, this statement: Electron delocalization contributes to making a carboxylic acid more acidic than an alcohol (which also contains an –OH group).
Draw and write in your notebook, then left-click here for an explanation.
There are two important resonance structures for acetate ion.
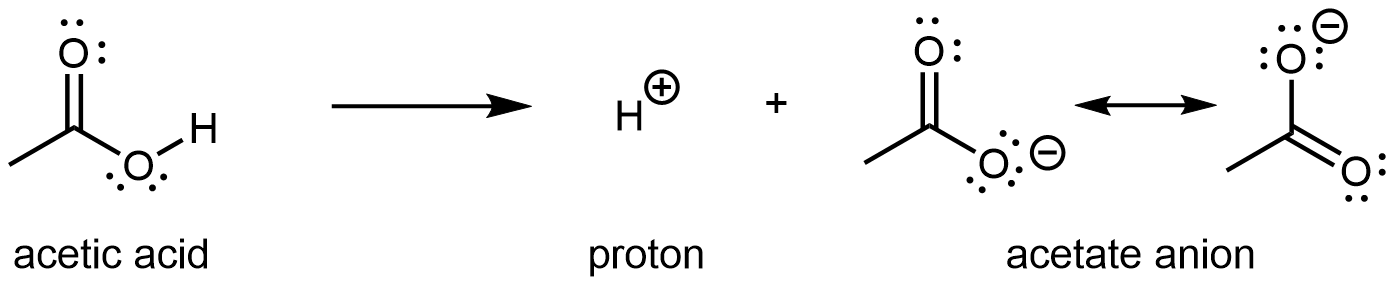
The two structures have equal weight in the resonance hybrid. They show that the π bond is delocalized to both C-O bonds, and moreover the excess negative charge of the anion is stabilized by delocalizing to both (highly electronegative) oxygen atoms. As a result of this, when the carboxylic acid loses its proton, the carboxylate ion that forms has lower energy than if the excess negative charge is localized to a single atom. In an alcohol, when an anion forms, a single Lewis structure is sufficient to describe the anion where the excess negative charge is on the single O atom. There is no resonance stabilization and so an alcohol is less likely to lose its proton.
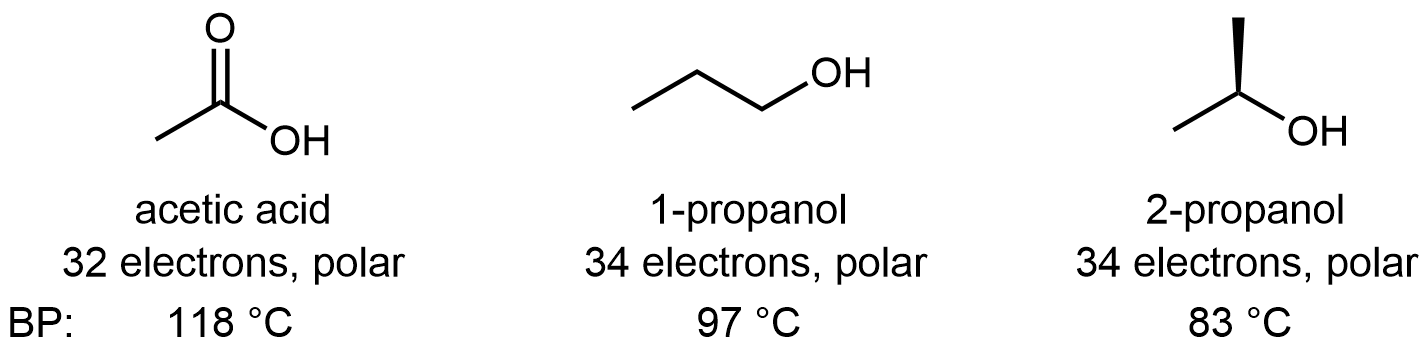
Molecules containing the carboxylic acid functional group are polar and can form hydrogen bonds. Pure acetic acid is called glacial acetic acid because its melting point of 16.6 °C is high enough that it can freeze in a cold laboratory. It is also quite thick and syrupy because the strong hydrogen-bonding attractions between molecules result in high viscosity. The acidity of the carboxylic acid group enhances the O-H···O hydrogen-bond strength, such that hydrogen-bonding between carboxylic acid molecules is usually stronger than between alcohol molecules. For example:
Amines
An amine functional group is a derivative of ammonia that contains one or more carbon-nitrogen bonds.
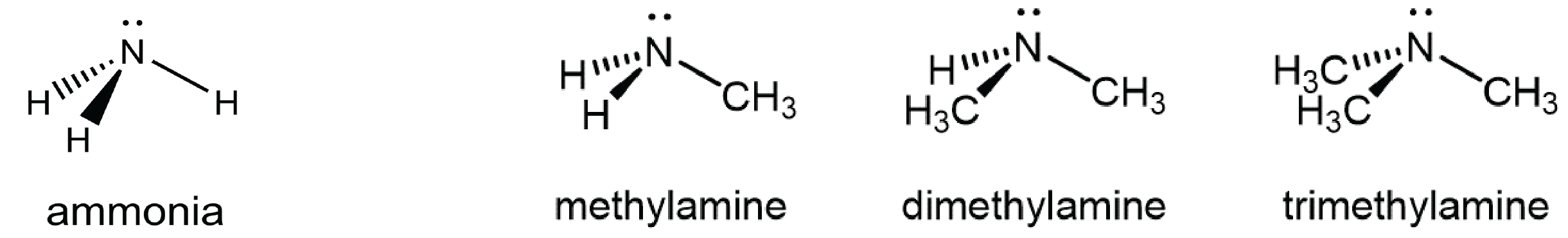
You can classify amine molecules by the number of C-N bonds they contain. In the above example, methylamine is a primary (1º) amine, dimethylamine is a secondary (2º) amine, and trimethylamine is a tertiary (3º) amine. In this figure methyl groups are shown but any R group, such as ethyl, could replace any of the methyl groups.
Like ammonia, amines are basic due to the lone pair on the nitrogen atom, and can undergo acid-base reactions to form protonated amine cations analogous to the ammonium ion NH4+:
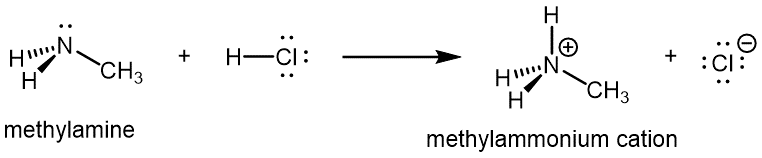
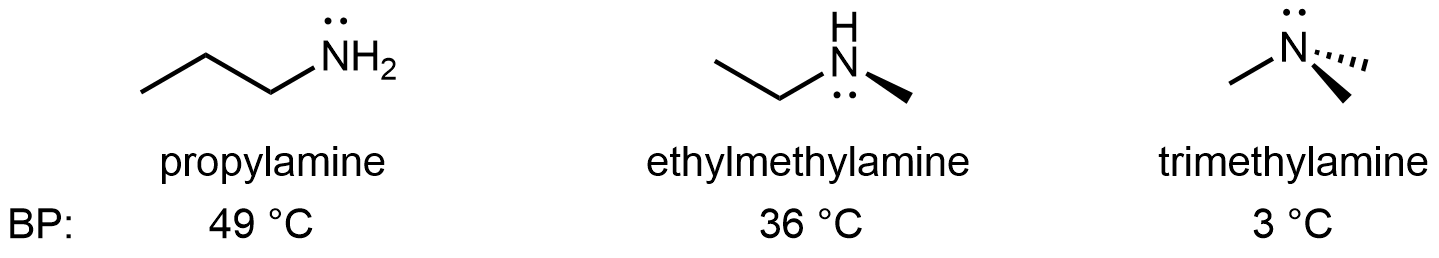
For amines, 1º and 2º amines are capable of hydrogen bonding due to the presence of a N-H bond and the lone pair on the N atom, but 3º amines cannot form hydrogen bonds. For example, for the three amine isomers shown below, the boiling point of the 3º amine is significantly lower than the 1º and 2º isomers.
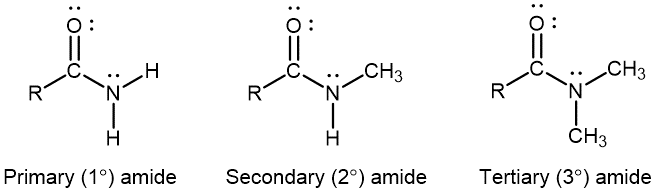
Amides
An amide functional group contains a nitrogen atom connected to the carbon atom of a carbonyl group. Like amines, amides can be classified by the number of carbon atoms bonded to the nitrogen:
Activity: Amines, Amides, and Resonance Structures
There is a crucial difference between amines and amides—there is an important resonance structure for the amide functional group that affects the molecular shapes, physical properties, and chemical properties of amides.
Draw a set of resonance structures for formamide (HCONH2). Based on the resonance structures you’ve drawn, determine the hybridization and local geometry of the N atom.
Draw and write in your notebook, then left-click here for an explanation.
The two resonance structures for formamide indicate that the nitrogen lone pair is involved in the π bonding network.
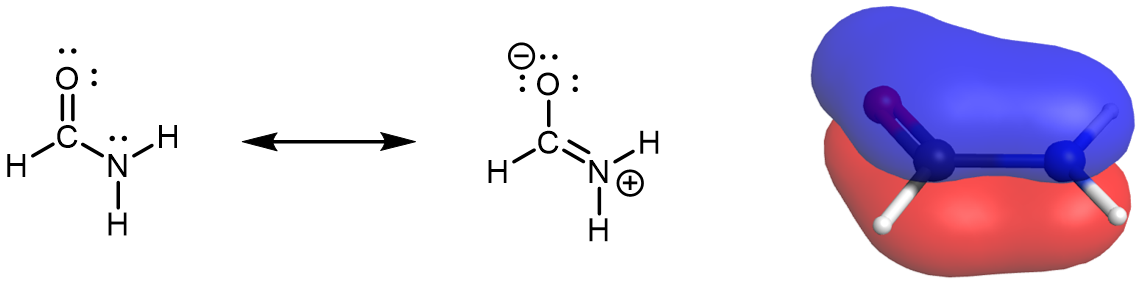
The delocalized π MO of formamide spans O, C, and N. Because the N lone pair is involved in the π bonding network, the lone pair must be in a p atomic orbital. Thus, the amide N atom is sp2 hybridized and has a trigonal planar local geometry. In fact, the whole formamide molecule is planar, as can be seen in this rotatable model.
Although the resonance structure with formal charges on O and N does not contribute to the resonance hybrid as much as the resonance structure without formal charges, it is crucial in understanding the chemical and physical properties of amide molecules. For example, the partial double bond character gives rise to a significant energy barrier for rotation about the C-N bond. And because the lone pair on the N atom is part of the π bonding network, the N atom in an amide is about 1010 times less basic than the N atom in an amine.
Additionally, because the lone pair on the N atom is part of the π bonding network, it is not available for hydrogen bonding. However, hydrogen bonds can still form in 1º and 2º amides between the N-H bond(s) and the lone pairs on the O atom.
Exercise: Structures of Amines
Exercise: Recognizing Functional Groups
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

