D7.2 When One “Bond” is not Enough
In space-filling models like those of ethene (C2H4) and ethyne (C2H2):
atoms appear connected, but there’s no visible indication of how many electron pairs are shared. These 3D models communicate shape and size, but not the details of electron arrangement.
That’s where Lewis structures come in. Lewis structures are built from electron bookkeeping: we assign valence electrons to atoms and connect them in ways that reflect their usual bonding patterns. Sometimes, after placing all single bonds, we find that all the valence electrons have not been used. When this happens, Lewis structures introduce multiple bonds, two or three shared electron pairs between atoms, to ensure all valence atoms are accounted for.
For example:
|
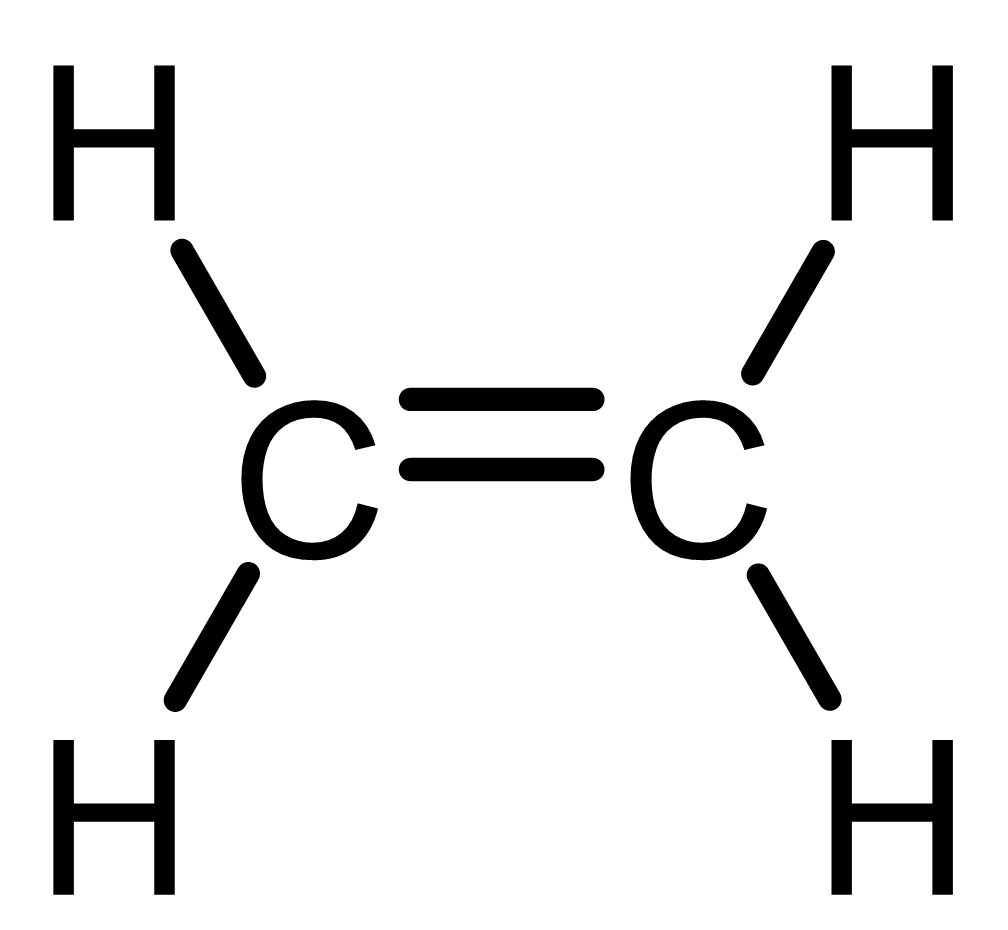 |
|
Activity: multiple bonds
Try drawing valid Lewis structures for ethane (C2H6), ethene (C2H4), and ethyne (C2H2). What patterns do you notice in how many pairs of electrons are shared between the carbon atoms?
Draw and Write in your notebook, then left-click here for an explanation.
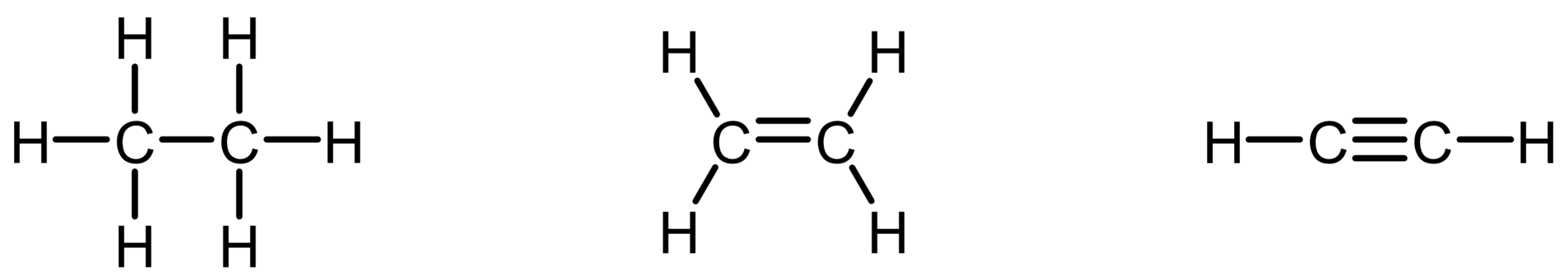
When you draw valid Lewis structures for ethane (C2H6), ethene (C2H4), and ethyne (C2H2), a key representational pattern emerges in how we depict the electron sharing between carbon atoms.
- In ethane, the two carbon atoms are connected by a single covalent bond, which we represent with one line; this corresponds to one pair of shared bonding electrons.
- In ethene, the carbon atoms are connected by a covalent bond involving two pairs of shared electrons, which we show using two lines in the Lewis structure. This is often referred to as a “double bond” in Lewis terms, though it’s still just a single bonding interaction between the two atoms—just with more electron density between them.
- In ethyne, the connection involves three shared pairs of electrons, represented as three lines in the Lewis structure. Again, while we refer to this as a “triple bond,” it’s important to remember that chemically, there is still one connection between the carbon atoms—just one that involves even more shared electron density.
What is abstracted in these Lewis representations is the fact that these is no “three separate bonds,” but rather one carbon–carbon bonding interaction involving more or fewer electron pairs. Also abstracted is the spatial arrangement of atoms: for example, ethane is tetrahedral at each carbon, ethene is planar, and ethyne is linear, but the Lewis structures do not capture that geometry.
So, Lewis structures use multiple lines to represent multiple pairs of bonding electrons, but chemically, there is just one bond connecting any two atoms. The number of lines simply reflects the number of electron pairs shared in that bond.
Even though single, double, and triple bonds are conventions used in Lewis structures, they reflect real differences in molecular behavior:
- As more electrons are shared, bond strength increases and bond length decreases.
Average bond strength Average bond length C–C 346 kJ/mol 154 pm C=C 598 kJ/mol 134 pm C≡C 813 kJ/mol 121 pm - Double and triple bonds often restrict rotation and influence reactivity.
- They affect electron distribution, which influences how molecules interact.
So while molecules just have bonds (electron-sharing interactions between atoms), our descriptions of those bonds (single, double, triple) are powerful tools for reasoning about structure, stability, and reactivity.
Exercise: Bond Lengths in Hydrocarbons
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

