D10.7 Electronegativity
Up to now, we’ve relied on Lewis structures to represent molecules: simple 2D models that show how atoms connect and where valence electrons are placed—either as shared pairs (bonds) or lone pairs. This approach helped us understand electron counting, bonding patterns, and formal charge, especially in stable molecules composed of C, H, O, N, and halogens.
But Lewis structures come with limitations. They assume electrons in bonds are shared equally between atoms. In reality, that’s not always true.
Electronegativity: Atoms Don’t Share Equally
Electronegativity (EN) is a property that helps explain the tendency of an atom in a molecule to attract electron density in a bond toward itself. You can think of it as a tug-of-war over bonding electrons. When two atoms with different electronegativities are bonded, the one with the higher electronegativity value pulls harder on the bonding electrons, creating an uneven distribution of electron density. We can represent this unevenness in three ways:
- A dipole arrow (→) pointing from the less electronegative atom to the more electronegative atom, with a crossbar at the positive end.
- The symbols δ⁺ and δ⁻ to indicate partial positive and partial negative charges.
- Electron density maps or diagrams that show areas of higher or lower electron probability.
Below are these three representations of uneven distribution of electron density applied to the molecule HF.
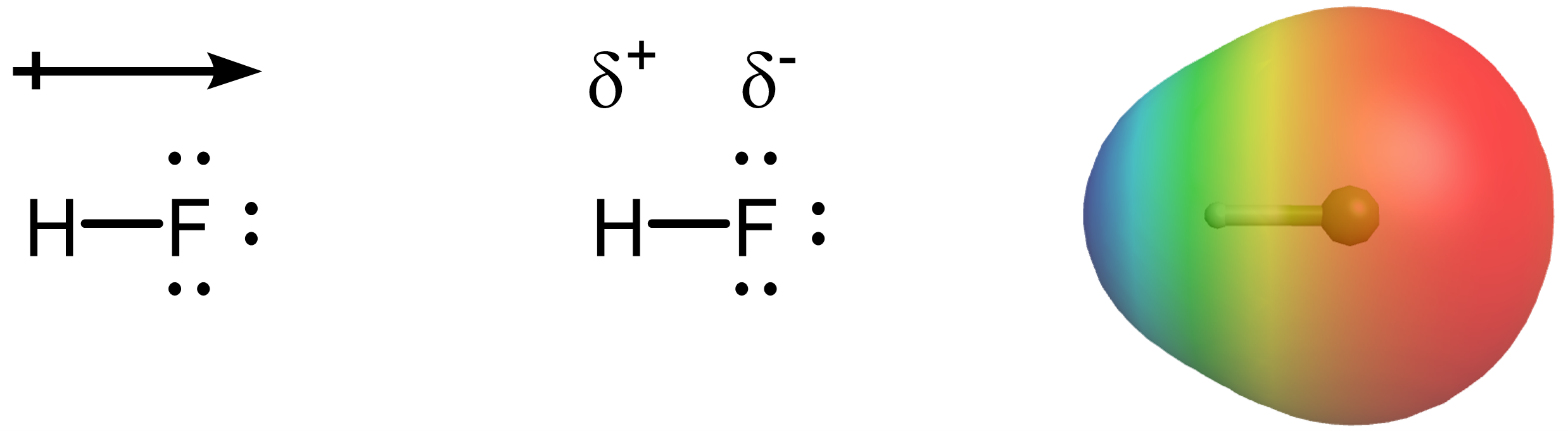
Periodic Trends in Electronegativity
Electronegativity is not randomly distributed across the periodic table. It follows predictable patterns, explained by trends in atomic structure.
From Polar Bonds to Polar Molecules
When two atoms of different electronegativities are bonded, we call the bond polar covalent. The more electronegative atom pulls more electron density toward itself, creating a dipole. For example, in H–Cl:
- Cl is more electronegative than H.
- Electrons in the bond are pulled toward Cl.
- Cl develops a δ⁻ partial charge; H develops a δ⁺ partial charge.
- The molecule has a bond dipole.
By contrast, in H–H or C–H bonds, the electronegativities are or are nearly equal, so the bond is nonpolar.
Activity
For each bond below, classify it as polar or nonpolar. Then draw a dipole arrow to indicate the direction of electron density shift, and assign δ⁺/δ⁻ symbols:
- C-F
- N-H
- Cl-Cl
Which of these bonds do you expect to be most polar? Why?
Draw and write in your notebook, then left-click here for an explanation.
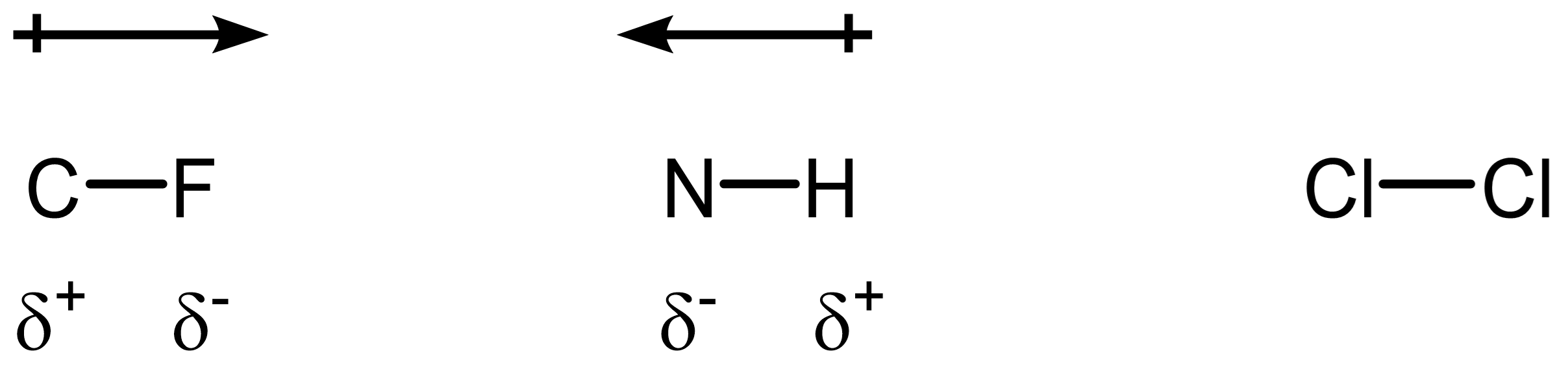
C-F is most polar, N-H is less polar than C-F, Cl-Cl is nonpolar. The difference in electronegativity is: C-F (1.4), N-H (0.9), Cl-Cl (0).
Formal vs. Partial Charge: Two Ways of Describing Charge Distribution
When building Lewis structures, we compute formal charges by assigning electrons based on simple rules: the shared electrons in bonds are split equally. This helps us determine whether a structure is plausible, but it does not always reflect reality.
In polar bonds, electrons are not shared equally, so we use partial charges instead. These reflect the actual electron density distribution, and they often disagree with formal charges. Let’s compare:
| Molecule | Formal Charges | Partial Charges | Explanation |
| HCl | Both 0 | δ⁺ on H, δ⁻ on Cl | Electrons pulled toward Cl, though formal charge is zero |
| NH4+ | +1 on N, 0 on H’s | δ⁺ on all the H’s, δ- on N | Positive charge is distributed across molecule |
Sometimes, formal charges are useful for counting electrons and evaluating stability. But when it comes to predicting polarity or charge distribution, partial charges offer a more realistic picture.
Why This Matters: Models and Stability
Charge distribution affects molecular stability and reactivity:
- Molecules with delocalized or spread-out charges are often more stable (we will see this in upcoming days!).
- Molecules where formal and partial charges align are often lower in energy.
- Mismatches (e.g., a Lewis structure placing a positive formal charge on an electronegative atom like oxygen) suggest a less plausible structure.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

